Traditional graphite-based anodes for Lithium-ion (Li-ion) batteries just don’t measure up in terms of charge life for the demands that are being put on them in today’s personal gadget applications. Silicon-based anodes as an alternative have demonstrated drastically improved charge life, but they begin to crack after a relatively few charge-discharge cycles.
As a result, much research has been devoted to finding nanomaterials that have the improved charge life of silicon but also the ability to withstand numerous charge-discharge cycles of graphite. Nanostructured silicon has been the nanomaterial that has been looked at the longest for achieving this dual aim.
Despite all the efforts, it wasn’t until last year that Yi Cui of Stanford and SLAC found a solution that consisted of a double-walled silicon nanotube coated with a thin layer of silicon oxide. This design was capable of storing 10 times more charge than graphite anodes and could survive 6000 charge-discharge cycles.
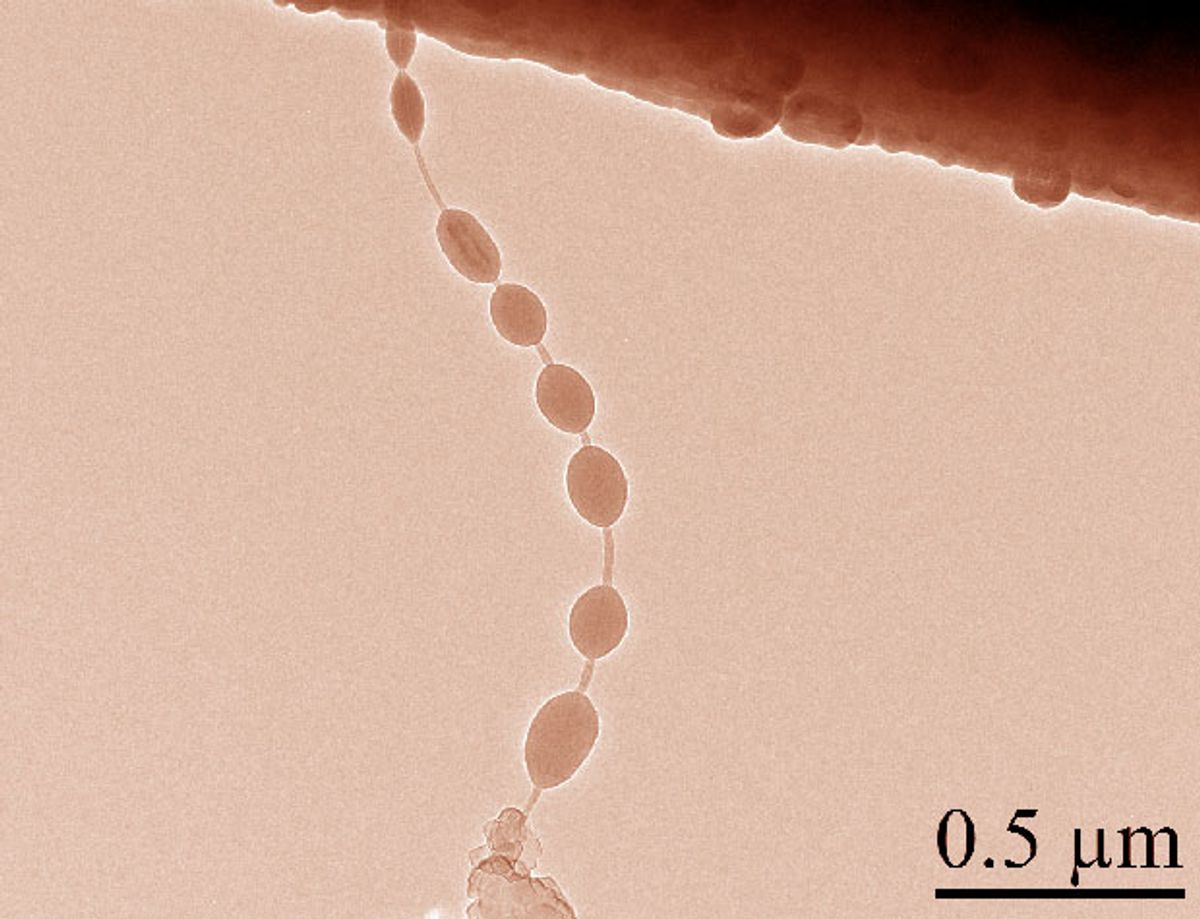
The research, which was published in the journal ACS Nano (“A Beaded-String Silicon Anode”), attached a molecule sometimes used in food flavoring to carbon nanotubes less than 50 nanometers wide. After flooding the molecule and carbon nanotube with silicon gas, the molecule caused beads of silicon to grow on the nanotube.
In their tests of the “nano beads on a string”—as the structure has been dubbed—they found that when it was charged with lithium ions the beads grew like a flexible balloon. When the ions left the nanobeads there was no cracking or breaking. A demonstration of this can be seen in the video below:
This is fairly preliminary research and it’s hard to tell whether it will ever develop far enough to mount a real challenge to the structure that Cui developed last year, or even the commercial aspirations of California Lithium Battery Inc. But just from the look of it, it appears to be a nanostructured silicon that self assembles in a fairly simple process. Sometimes simple processes win out over superior functionality.
Image: University of Maryland NanoCenter
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.