In the world of nanomaterials, carbon nanotubes and graphene have taken a large share of the attention. But in terms of real-world impact, it’s hard to ignore the role of quantum dots. For the past couple of years, these semiconductor crystals appear to have taken over the displays market. However, quantum dots are vying for a major role in a number of other applications in which their photoluminescence is key, such as photovoltaics and medical applications.
But in order to get quantum dots to do the things we want them to do, they sometimes need to be doped with impurities in order to change their properties. This is where things get tricky: Quantum dots are so small that the dopants tend to wiggle back out of them. The good news: One of the more commonly used methods for introducing these other molecules to quantum dots—something called “click chemistry”—joins molecules together in a way that is fairly easy and results in easily removable byproducts. The bad news: The click chemistry technique that is the most appealing for quantum dots uses copper to catalyze the reaction. But the copper ions end up stripping the quantum dots of their photoluminescence.
Now researchers at the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) in Warsaw, and the Faculty of Chemistry of the Warsaw University of Technology (FC WUT) have developed a method that keeps the click chemistry’s copper catalyst, but prevents it stripping the quantum dots of their photoluminescence. The key turns out to be improving the quality of the quantum dots.
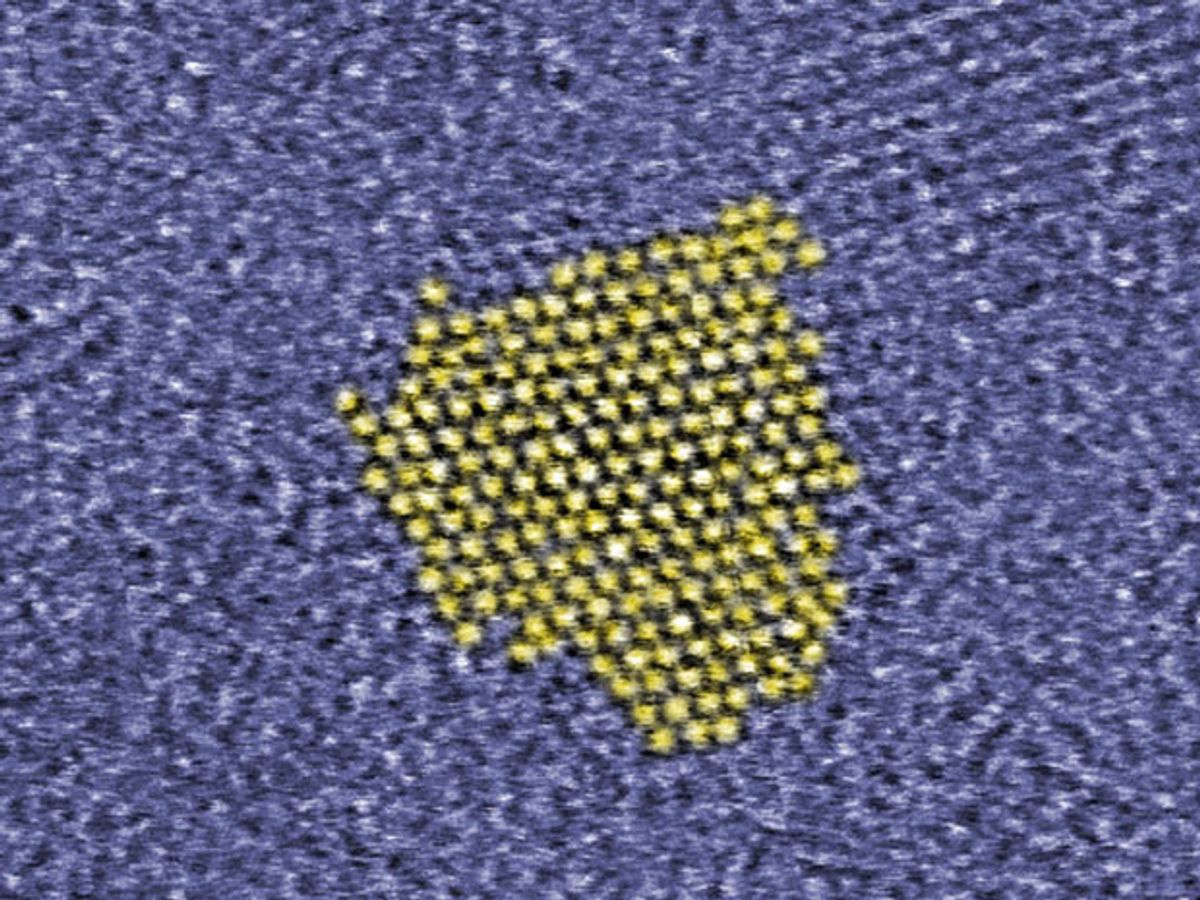
In research described in the journal Chemical Communications, the Polish researchers produced zinc-oxide quantum dots that have outer shells protecting them from the copper ions. Currently, quantum dots are produced in a sol-gel process that results in some of them having a leaky protective shell that allows the copper ions to come in direct contact with the surface of the quantum dots.
Malgorzata Wolska-Pietkiewicz, a PhD student at FC WUT, who participated in the research, described in a press release the key quality of the quantum dots produced in this process:
“Nanoparticles produced by our method are crystalline and all have almost the same size. They are spherical and have characteristics of typical quantum dots. Every nanoparticle is stabilized by an impermeable protective jacket, built of organic compounds, strongly anchored on the surface of the semiconductor core. As a result, our quantum dots remain stable for a long time and do not aggregate, that is clump together, in solutions."
These results represent the first time that it has been possible to produce quantum dots from organometallic precursors in a way that helps them to retain theier valuable optical properties after being subjected to copper-catalysed click reactions, according to Janusz Lewinski, the lead researcher and a professor with appointments at both schools.
The researchers believe quantum dots produced in their newly developed process will prove to be particularly useful in medical imaging technologies because they are non-toxic, don’t clump together, can be fairly easily combined with other chemical compounds and through it all retain their full photoluminescence. Combining them with biologically active materials will allow the labeling and long-term tracking of cells and entire swaths of living tissue.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.