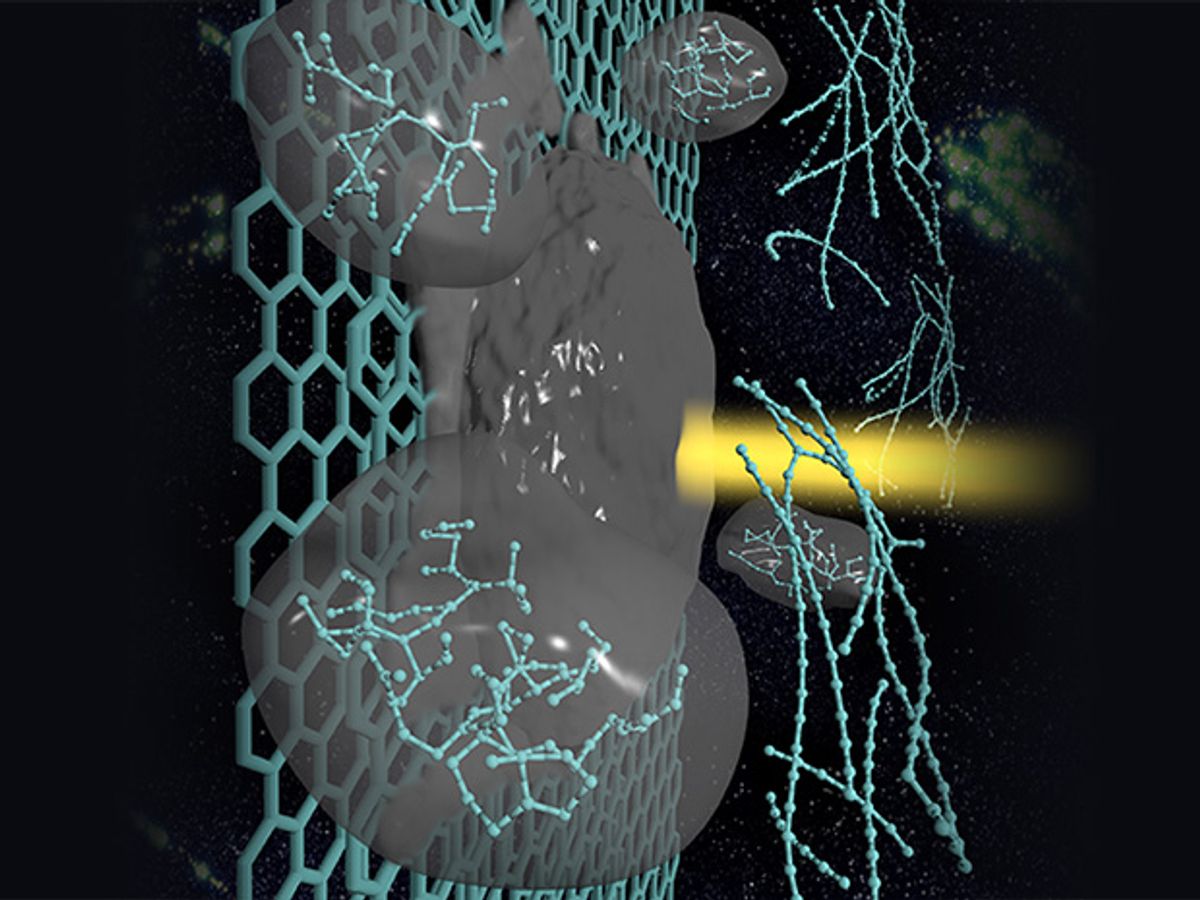
A couple of years ago, a material dubbed carbyne—which is a chain of carbon atoms held together by either double or alternating single and triple atomic bonds—was awarded the title of the world’s strongest material. Later, scientists also demonstrated that it has the unusual property of being able to change from being a conductor to an insulator when it’s stretched by as little as 3 percent.
Both are great properties to have. But thus far carbyne has proven nearly impossible to make in the real world. As a result, its exploits have remained firmly planted in the realm of computer models. While carbyne has been found in highly compressed graphite, and it has even been synthesized at room temperature, no one has devised a way to produce the material in bulk.
While researchers at Lawrence Livermore National Laboratory (LLNL) looking at carbyne’s properties in computer models and discovering some new ones that should benefit nanoelectronics, they discovered a potential avenue for producing the material.
In research published in the Journal of Physical Chemistry, the LLNL researchers demonstrated through computer models that it was possible to form carbyne fiber bundles by melting graphite with a laser.
“There’s been a lot of speculation about how to make carbyne and how stable it is,” said Nir Goldman, an LLNL scientist, in the press release. “We showed that laser melting of graphite is one viable avenue for its synthesis.” And depending on how you cook the graphite, the resulting carbyne could have applications, including tunable semiconductors or even hydrogen storage materials. Says Goldman:
Our method shows that carbyne can be formed easily in the laboratory or otherwise. The process also could occur in astrophysical bodies or in the interstellar medium, where carbon-containing material can be exposed to relatively high temperatures and carbon can liquefy.
Whether this technique can be scaled up to producing carbyne in bulk remains to be seen. But if it proves to be a viable method for carbyne synthesis, it does buoy hope for the material’s use in nanoelectronics, where it could perform some amazing feats such as adjusting the amount of electrical current traveling through a circuit, according to the user’s need.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.