The role of nanotechnology in next-generation fuel cells has a bit of a shaky past. There were initial thrills nearly a decade ago, when carbon nanotubes appeared to store hydrogen at an enormous ratio of 50 percent of their total weight, which, unfortunately, was later reduced to the rather sobering ratio of 1%wt in practical applications. And, of course, lest we forget, there was NEC’s promise, over a decade-and-a-half ago, of a fuel-cell-powered laptop enabled by nanomaterials. It’s not a spoiler to say it never came to fruition.
Since then, the hype around nanomaterials has largely moved on from improvements in the fuel cell itself, to efforts to achieve artificial photosynthesis for producing hydrogen.
Now researchers at the Korean Advanced Institute of Science and Technology (KAIST) have bucked that trend and gone back to trying to exploit nanotechnology to make a better fuel cell. The Korean researchers have devised a way to make the more cost-effective hydrocarbon membranes used in today’s proton exchange membrane (PEM) fuel cells even stronger and longer lasting.
PEM fuel cells are attractive for use in vehicles because they operate at low temperatures. However, they do have weaknesses. The KAIST researchers made the breakthrough while attempting to address one of these weaknesses. In the PEM fuel cell, the the permeable catalyst membrane that separates the two chemical compartments serves as a kind of electrolyte. One side of it is bonded to the positive electrode, and the other side, to a negative one.
The problem is that the membrane can be made from either a perfluorosulfonic acid–based polymer, which is very expensive, or from a hydrocarbon that is less expensive but doesn’t last very long.
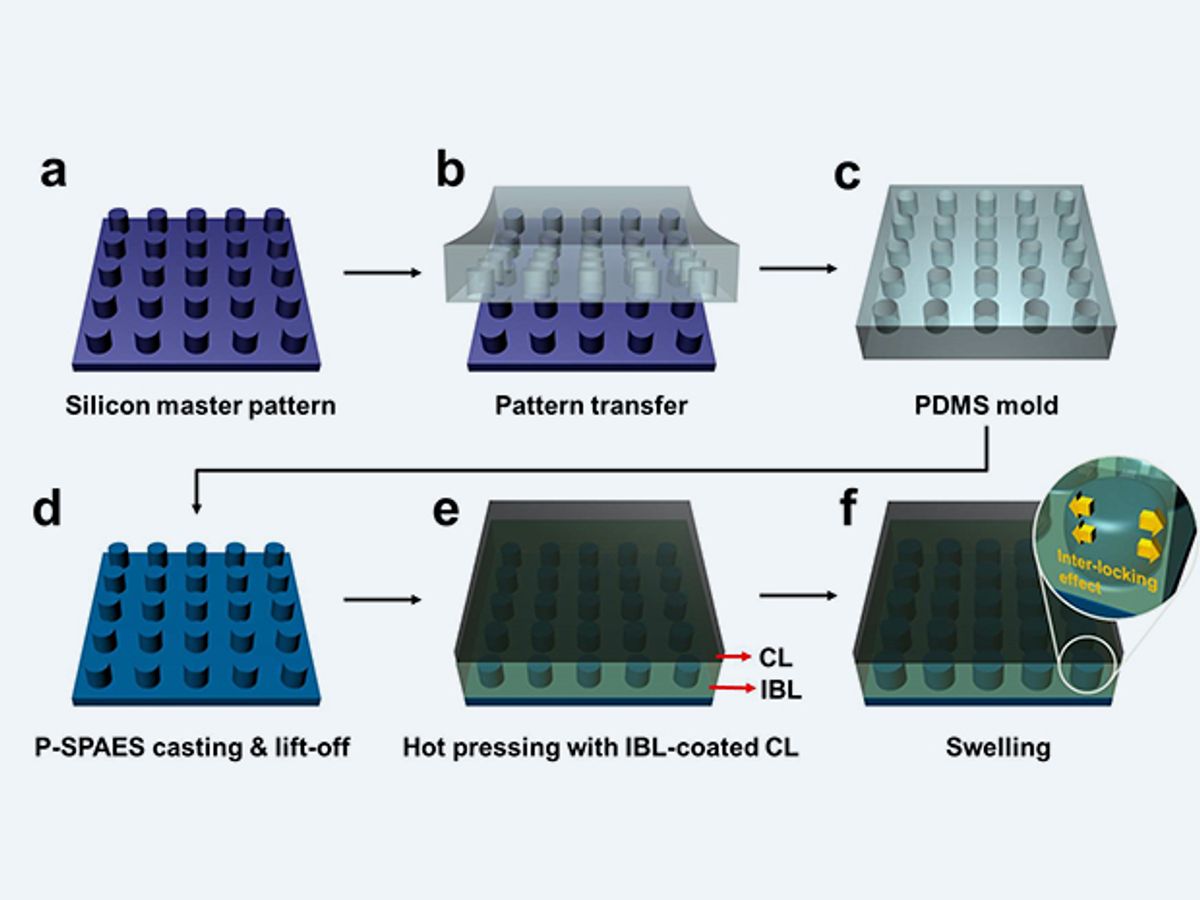
The KAIST researchers set out to strengthen the less expensive hydrocarbon membranes. To that end, the KAIST researchers have developed nanoscale fasteners that bond the membrane to the electrodes mechanically rather than chemically.
“This physically fastened bond is almost five times stronger and harder to separate than current bonds between the same layers,” said Professor Hee-Tak Kim of KAIST, in a press release.
The nanoscale fasteners were fabricated by creating a mold for tiny pillars on the surface of the hydrocarbon membrane. When the interface between the membrane and the electrodes begins to heat up, the pillars begin to push into the softened surface of the electrode. When the interface cools and absorbs water, the connection between the pillars and the electrodes begin to set and the mechanical bond is established.
While this production method provides a much sought after way to fabricate fuel cells that are less expensive, more efficient, and easier to manufacture, it may have applications beyond fuel cells. The researchers envision this approach being useful for any application that employs hydrocarbon membranes, such as rechargeable “redox flow” batteries.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.