While the prospect of aluminum-ion batteries may have received a lift recently, the workhorse battery for both our handheld electronic devices and our electric vehicles remains the ubiquitous lithium-ion (Li-ion) battery.
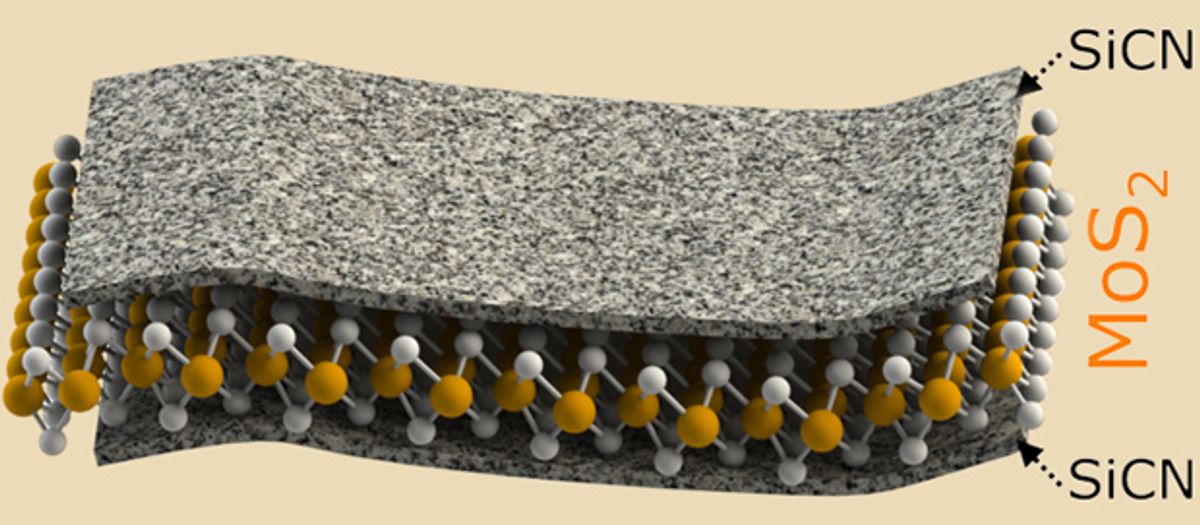
And now, researchers at Kansas State University (KSU) have taken a fresh look at the venerable Li-ion battery: Using the two-dimensional material molybdenum disulfide (MoS2) on its electrodes, they say, may dramatically boost its storage capacity. What they have come up with is a hybrid material that combines MoS2 with silicon carbonitride (SiCN); it can store double the charge of electrodes using MoS2 on its own.
Last year, KSU researchers, led by Gurpreet Singh, demonstrated the effectiveness of MoS2 in overcoming some of the key shortcomings of sodium-ion batteries. It appears Singh and his colleagues have turned their attention to addressing the issues of cycling stability and capacity retention as seen with previous research in which bulk MoS2 was used.
In research published in Nature’s Scientific Reports, the KSU team observed that MoS2 sheets that had been wrapped in silicon carbonitride could store twice as much lithium as pure MoS2. The reason may be the same mechanism underlying the issues the researchers faced when studying sulfur-ion batteries: sulfur gets into the electrolyte, reducing its capacity.
"This kind of behavior is similar to a lithium-sulfur type of battery, which uses sulfur as one of its electrodes," Singh said in a press release. "Sulfur is notoriously famous for forming intermediate polysulfides that dissolve in the organic electrolyte of the battery, which leads to capacity fading. We believe that the capacity drop observed in molybdenum disulfide sheets is also due to loss of sulfur into the electrolyte."
By wrapping the MoS2 in silicon carbonitride, which is a ceramic material capable of withstanding high temperatures, the MoS2 is prevented from giving off its sulfur atoms and creating the polysulfides that would eventually dissolve in the electrolyte.
"The silicon carbonitride-wrapped molybdenum disulfide sheets show stable cycling of lithium-ions irrespective of whether the battery electrode is on copper foil-traditional method or as a self-supporting flexible paper as in bendable batteries," Singh said in the release.
In a full comparative table provided in the open-access research paper, you can see the big difference in durability between the SiCN-MoS2 hybrid versus the MoS2 on its own. The MoS2 material on a traditional electrode starts with a charge capacity of 595 milliampere-hour per gram, and after just 20 cycles, falls precipitously to just 16 mAh/g. Meanwhile the SiCN-MoS2 on a traditional electrode, starts at 572 mAh/g and after 20 cycles is still at 104.8 mAh/g. When a SiCN-MoS2 paper electrode is used, the new formulation’s numbers are even more impressive, starting at 623.5 mAh/g and registering 417.8 mAh/g after 20 cycles.
In further research, Singh intends to see how this battery design might function in an actual handheld device. The key to this line of research will be testing its ability to store energy over the course of hundreds of charge-discharge cycles.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.