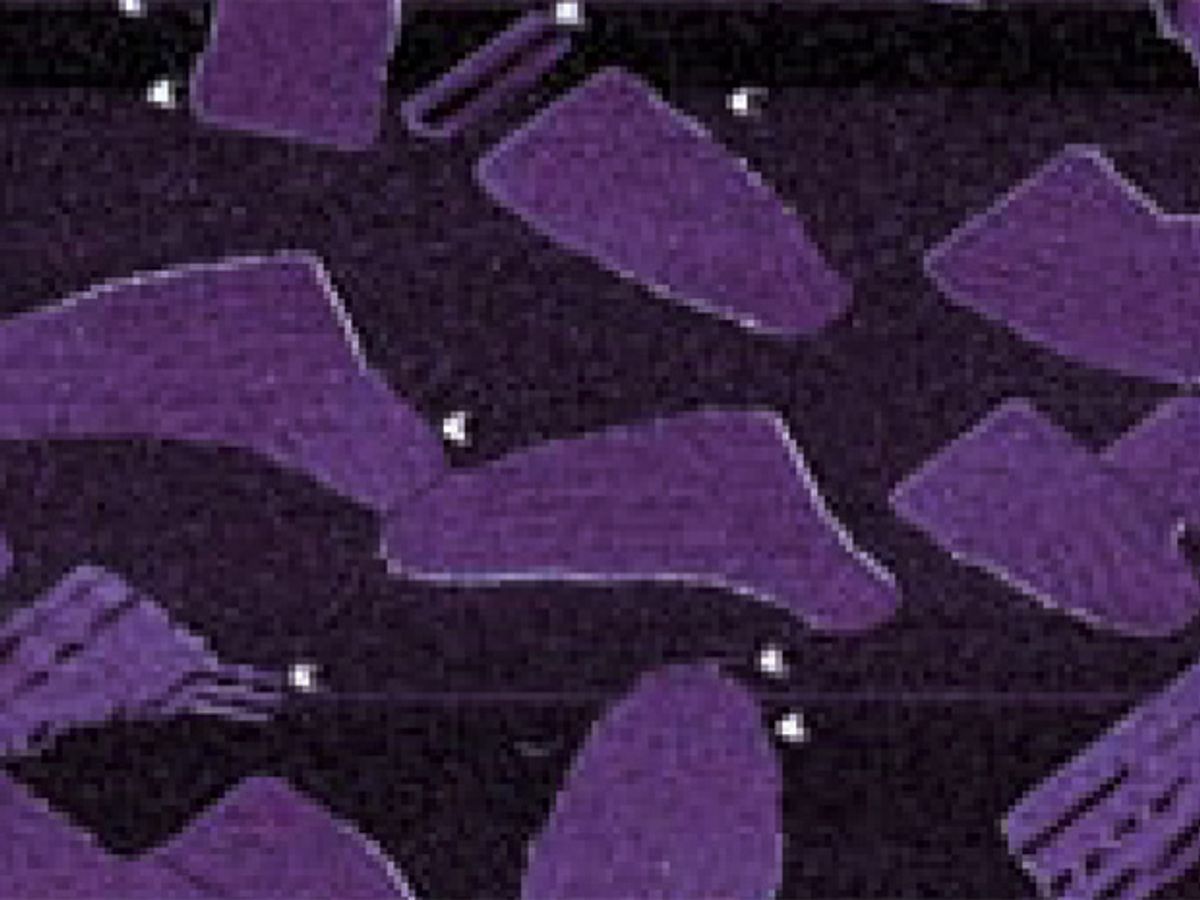
Future electronic devices and batteries could become faster and more energy efficient by harnessing new 2-D sheets of electrically-conductive materials. The latest example of such materials comes in the form of newly-created sheets of boron atoms, called borophene, that could outperform even graphene as an electrical conductor in 2-D form.
Carbon-based graphene sheets have long been hailed as a potential replacement for the silicon commonly used in computer chips and electronics. But a similarly flat sheet of borophene just one atom thick should theoretically outperform graphene. An international research team from the U.S., China and Russia has finally overcome the steep challenges in making borophene and demonstrated the first example of such 2-D boron sheets. Even better, their approach to making borophene can be easily replicated by other labs around the world—opening the door to both scientific research and technological development involving borophene.
“We found an easy and inexpensive technique which allows the broader scientific community to create borophene,” says Andrew Mannix, a Ph.D. candidate in materials science and engineering at Northwestern University and a researcher at Argonne National Laboratory.
The inexpensive technique involves growing the borophene inside an ultrahigh vacuum chamber that keeps the experimental conditions clean and devoid of possible contaminant materials. Mannix and his colleagues chose to use silver as an underlying “substrate” layer for borophene to grow on because silver does not readily react chemically with boron.
An electron beam evaporator heated the boron material to the point where it evaporated and eventually deposited on the silver. Experiments showed that the borophene deposits grew the best when the silver’s temperature was maintained at about 550 degrees C, but growth could be achieved at temperatures ranging between 450 degrees C and 700 degrees C. The research is detailed in the 18 Dec 2015 issue of the journal Science.
Researchers have traditionally made boron-based structures by using chemical vapor deposition. In the case of boron, that technique requires use of an expensive gas called diborane that also represents a neurotoxin. The risks of working with such gas require a costly, protective lab setup that is not always commonly available. By comparison, the newer approach of growing borophene on silver in an ultrahigh vacuum chamber only requires equipment that can be found at pretty much any university.
The newly-created borophene sheets didn’t always look the same—they ended up taking the form of two distinct phases. Higher deposition rates and lower temperatures generally created a “homogenous phase” sheet of borophene that is fairly even and flat. On the other hand, low deposition rates and higher temperatures tended to create “striped-phase nanoribbons” that resemble tightly bunched crinkles in the typically flat borophene sheet.
Researchers don’t yet know which phase of borophene might prove most useful in future technological applications. But the early experiments already show they could engineer the system to get a larger fraction of one or the other phase if they so desire, Mannix says.
The creation of borophene opens the door for more researchers to begin investigating certain unique properties of the material. Theoretical calculations suggest the “striped-phase” borophene will actually have very different characteristics depending on which direction you face looking across the surface of the sheet.
For example, the borophene should prove more metallic in the direction parallel to the “crinkles” in the sheets, as opposed to going in the perpendicular direction cutting across the crinkles. The possibility of borophene acting as a one-directional metal could have “technological relevance” for future electronics, Mannix says.
Similarly, calculations suggest that borophene should prove stronger than graphene in the direction parallel to the crinkles. That’s notable given how graphene represents the strongest known material to date. But the borophene would be weaker than graphene in the perpendicular direction because the crinkles would exhibit an accordian effect.
Plenty of challenges remain in terms of working with borophene. The researchers want to see if the borophene sheets hold up when separated from the silver, or if the sheets end up buckling and become more deformed.
Borophene also remains somewhat unstable when exposed to air. The researchers discovered that the 2-D boron sheets begin undergoing chemical reactions when pulled out of the ultrahigh vacuum chamber and exposed to open air—a process that begins within hours if not sooner. By comparison, bulk boron material will remain stable for a few days before it begins undergoing chemical reactions with the air.
One possible solution to such instability comes from encasing the borophene within a protective silicon coating. But the researchers hope to find other material coatings or chemistry solutions that make borophene more stable in the long run.
The study received funding from the Department of Energy’s Office of Basic Energy Sciences and took place primarily at the Center for Nanoscale Materials at Argonne National Laboratory. But the overall research came together as an international collaboration between U.S., Chinese and Russian researchers.
The U.S. researchers had initially pursued their silver-based approach to making borophene on their own, given their previous experience in growing graphene on silver. They eventually reached out to Chinese and Russian researchers who provided additional theoretical guidance on using a silver substrate to grow borophene. In a good example of great minds thinking alike, the U.S. team had already been following the right track in the first place.
“It’s rare that the experimental stuff works on the first try, so we really lucked out there,” Mannix says.
Jeremy Hsu has been working as a science and technology journalist in New York City since 2008. He has written on subjects as diverse as supercomputing and wearable electronics for IEEE Spectrum. When he’s not trying to wrap his head around the latest quantum computing news for Spectrum, he also contributes to a variety of publications such as Scientific American, Discover, Popular Science, and others. He is a graduate of New York University’s Science, Health & Environmental Reporting Program.