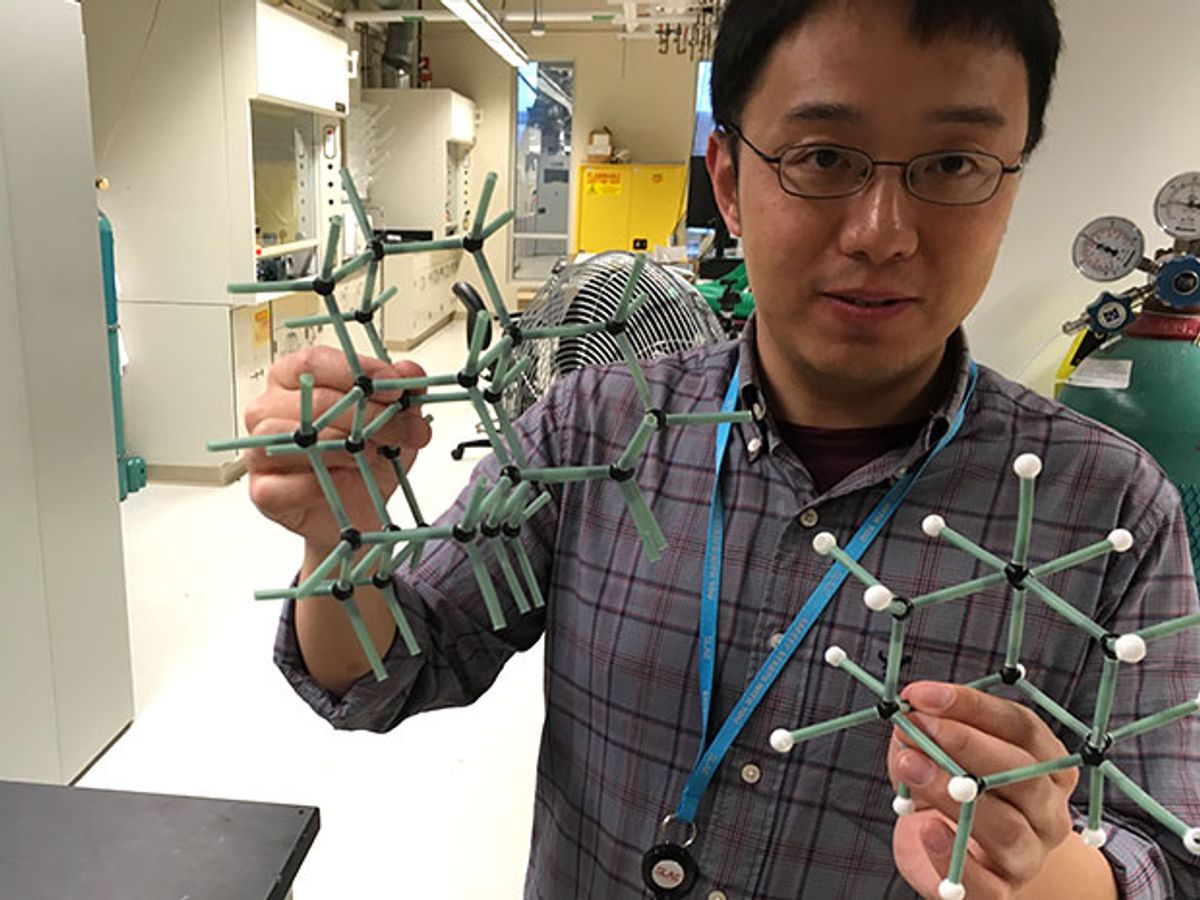
Ever since Jeremy Dahl of Stanford University first isolated the molecules known as “diamondoids” from crude oil in 2003, the material science community has been fascinated with their potential. Diamondoids, which are both the smallest and purest form of diamond, have a unique set of properties that have led scientists to consider their use in applications including quantum computation and enabling so-called diamondoid mechanosynthesis (DMS), in which diamondoid structures are built using a programmable molecular positioning approach.
While DMS may still be a long ways off, researchers at Stanford and the U.S. Department of Energy’s SLAC National Accelerator Laboratory in Menlo Park are continuing to experiment with the material. They have developed more short-term applications for the molecule, while still keeping their eyes on the horizon for its long-term potential.
Hao Yan, a postdoc with the Melosh Research Group at the Stanford Institute for Materials and Energy Sciences (SIMES), reported late last year in the journal Nature Materials that diamondoids could help control the self-assembly of nanowires, promising a kind of template for creating a variety of materials with unique properties.
“What we were trying to do in growing the nanowires with the help of the diamondoids is create electronic systems where the critical dimensions are in the nanometer scale,” said Yan last week when we visited him at the lab where he synthesized the material. “We are not only trying to do that—because that's been there for decades—but we are also trying to define the structure at atomic precision level.”
Yan believes that fine-tuning nanowires this way can lead to a previously unseen ability to control their electronic properties, including their band gap, dispersion, and how the electrons transfer through their structure.
The diamondoids are critical in this aim because these molecules are very rigid and they have particular surface properties that make them interact very strongly. The interaction of these molecules takes the form of van der Waal forces that are traditionally characterized as weak interactions compared to chemical bonds. In the video below, Yan describes the diamondoid molecule and some its unique bonding properties.
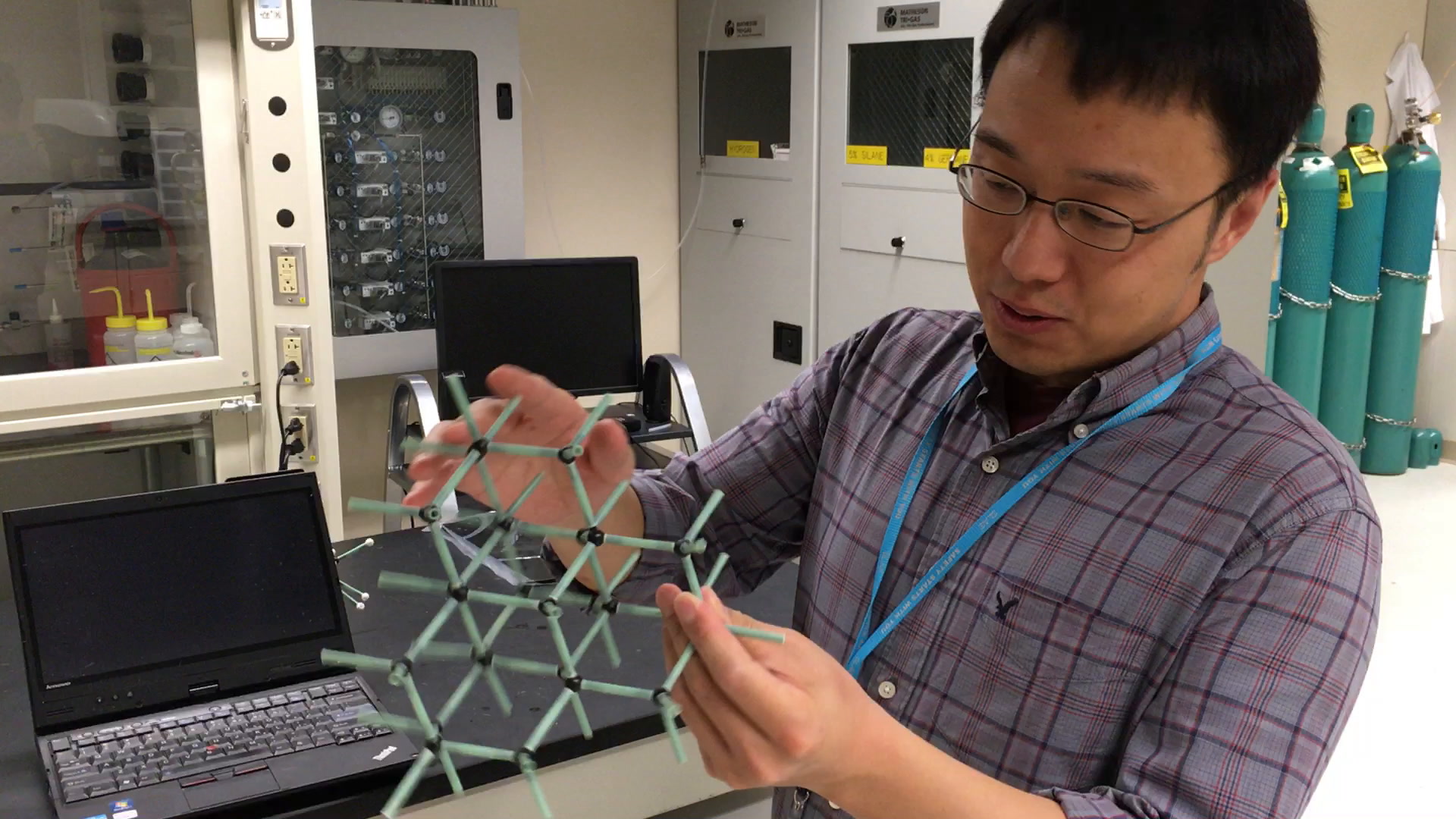
Over the years, the Stanford researchers have found that that these so-called weak interactions, at least in the diamondoids, are so strong that they're comparable to a chemical bond. The implication has been that researchers might be able to harness this strong attraction between the diamondoids in order to modify how other atoms attach to them and how those other atoms bond to each other.
“We discovered that once you use [diamondoids] as an assembly template, you can grow these ultrathin nanowires that are only 3 atoms in their circumference,” says Yan. “[The result is] precise control of how these atoms bond to each other—to the diamondoid and also to each other.”
Yan starts with diamondoids in a solvent like toluene, and another vessel containing copper sulfate dissolved in a solvent like water or ethylene glycol. When one of the solutions is added to the other, they do not mix; they form a layered solution. It is at the interface between the two solutions that the interesting stuff happens.
“After a day or two, you begin to see the nanowires form at the interface of these two liquids, which is pretty amazing, said Yan. “It's almost like these diamondoids know where they want to go, but it's all because of the interaction between the metal, the sulfur and the diamondoids that determines how the structure will assemble into nanowires.”
This line of research, says Yan, is branching off in two directions. The first aim is to expand the range of materials being tested. For instance, the researchers are looking at iron-based materials that could form superconductors when combined with the diamondoids.
The other direction is more theoretical. They want to understand why these structures grow into certain shapes. For example, in the paper describing the nanowires, the researchers demonstrated an initial understanding of why copper sulfide in combination with two different types of diamondoids guided the structure to grow either into a nanowire or a nanoribbon.
“Ideally, we want to get to the point where I can say give me a diamonoid and give me a metal and a chalcogen and I can tell you without doing any experiments what kind of structure will come out of this assembly,” says Yan.
Ask any material scientist, and they’ll tell you that it’s extremely challenging to predict what a material’s crystal structure will be based on two atoms. That’s because of the vast amount space that these atoms can explore. But diamondoids are poised to change the game.
“These properties of diamondoids eliminate a lot of the possibilities of space we can reach,” said Yan. “So we are already narrowing down a number of the possibilities.”
The ultimate aim would be a predictive model that would allow high-throughput methods for screening hundreds of thousands of materials and seeing which ones researchers should target. Yan concedes that we’re not likely to see such a model for at least five to 10 years.
However, there are more short-term applications for the diamondoids that Yan and his colleagues are working on.
One line of research that the researchers are working on is the development of an electron gun. Electron guns are used to create a stream of electrons. They are used in electron microscopy to irradiate a sample so that you can resolve the structure. They are also used in synchrotrons, one of the key tools at the SLAC labs.
The problem with the electron guns that are commercially available now is that they have a very broad range of energy, or electron wavelength color. You either have to sacrifice the resolution or use a filter that cuts out a very thin slice of this wide spectrum. The consequence of that is that you're losing a lot of brightness. It forces a trade off between how many electrons you use and how pure the beam is in terms of its energy.
“What we found was with these tiny diamondoids is that just a monolayer of this material works as an energy funnel. It takes this broad range of electrons with very different kinetic energies and bundles them up together such that it when exits the molecule, you have thousand-fold shrinking of this distribution,” says Yan. “This means that what conventionally has to be done by very complex filtering systems to create this monochromatic electron beam, you can now do with just a monolayer of this diamondoid material. That’s a very powerful thing.”
Another application area for diamondoids that Yan characterizes as being further along in development than some of the others is to use the diamondoids to synthesize ultra-thin and ultra-pure diamond films.
In a number of applications—especially for quantum computation—diamonds are quite useful. If you have a certain kind of defect in diamonds, it can act as a storage bit. The success of this data storage strongly depends on how pure the diamond structure is. But today’s methods for creating diamond films don’t lead to a high purity.
“Now, with diamondoids, these are the purest diamond that we can find,” says Yan. “The thinking there is that if you start from an extremely pure seed, you can grow extremely pure diamond. So we are exploring that direction and trying to create ultra-pure diamond films. Once you have ultra-pure diamond, then you can control the introduction of defects that serve as the storage bytes. If you have random defects already in there, then you lose that control.”
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.