Idiot-Proofing the Defibrillator
How a device that shocks a failing heart back to life became one of the greatest engineering success stories in medicine
At its best, the human heart is a supple machine. When the hydraulics work as they should, an electric current rhythmically moves blood through the heart’s four chambers. Electric impulses travel along specialized fibers and then dart from cell to cell, causing the muscle fibers to contract and relax as regularly as a second hand ticking around a clock face. When they contract, the muscle fibers create high-pressure regions that push open the heart’s valves. Blood pours out of one chamber into another, and just ahead of the blood travels the current, methodically exciting the right fibers and cells in sequence.
But when things go wrong, that current can incite incredible chaos. If a heart is stricken by ventricular fibrillation—a common type of cardiac arrest—then its once-orderly conduction devolves into a scattering of impulses. These impulses travel through the heart as little wavelets, causing unsynchronized tightening and releasing of the muscle fibers in the ventricles, the two lower chambers of the heart. Without synchronization, blood flow ceases; starved of oxygen, other organs rapidly begin to fail. Within 10 minutes, the victim will almost certainly die.
About one-fourth of all deaths in the developed world can be attributed to cardiac arrest—an astounding figure, and one that now has a chance to drop, in no small part because of the automated external defibrillator. AEDs are designed to shock a heart that’s in ventricular fibrillation back into a healthy rhythm. The device is now so easy to use that even an untrained bystander can administer this time-critical and highly effective medical procedure. AEDs, which fit into a case the size of a lunch box [see “Simply Saved”], can now be found in hundreds of thousands of public places, including office buildings, transportation hubs, and gyms; they’ve also been installed in police cars, in schools, and even on the International Space Station. As the price continues to drop—units can sell for as little as US $1000—some experts are urging people at high risk for cardiac arrest to keep an AED in their homes, just in case.
The AED’s widespread dissemination represents one of the greatest engineering success stories of the last few decades. In just 20 years, improvements in defibrillator design—in the efficacy of the waveform that delivers the electric shock, the way that the unit’s energy is stored and delivered, and the AED’s overall ease of use—have made it so that a layman can operate it with little more than a quick tutorial.
You’re meeting with a middle-aged coworker who suddenly slumps over. You have no idea what’s wrong with him, but cardiac arrest seems likely. Fortunately, you’ve had basic training in how to respond to an emergency, so you snap out of your initial state of shock and call for emergency medical assistance.
On average, the wait for an ambulance in populated areas of the United States is about 11 minutes. From your first-aid training you know that a cardiac-arrest victim’s chance of survival drops about 10 percent with every passing minute, which doesn’t bode well for your coworker. You remember seeing an AED hanging on a wall nearby, and you dash off to retrieve it. Defibrillator in hand, you struggle to keep cool as you try to recall how this strange device works. You’re under immense time pressure in an unfamiliar situation where every second counts.
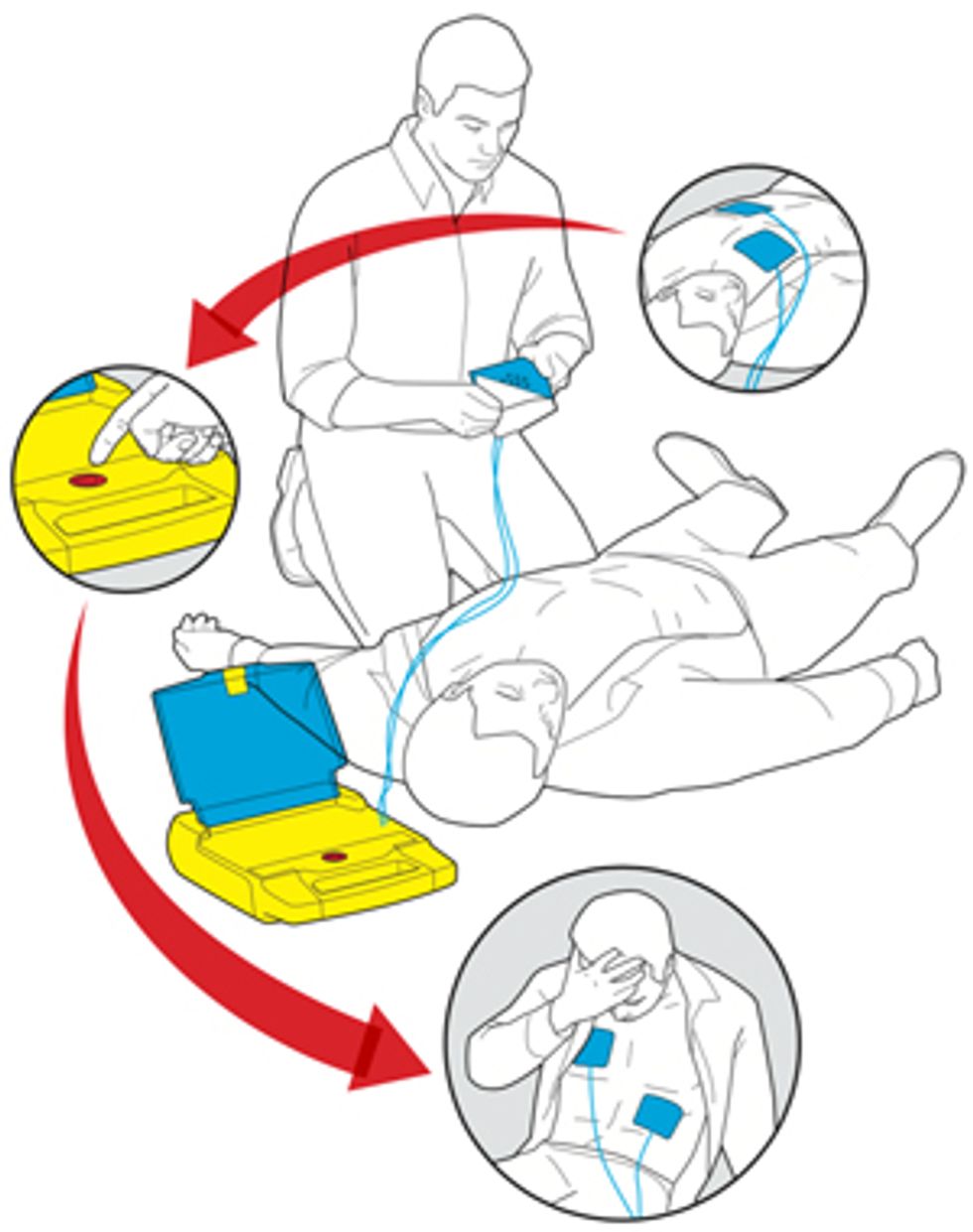
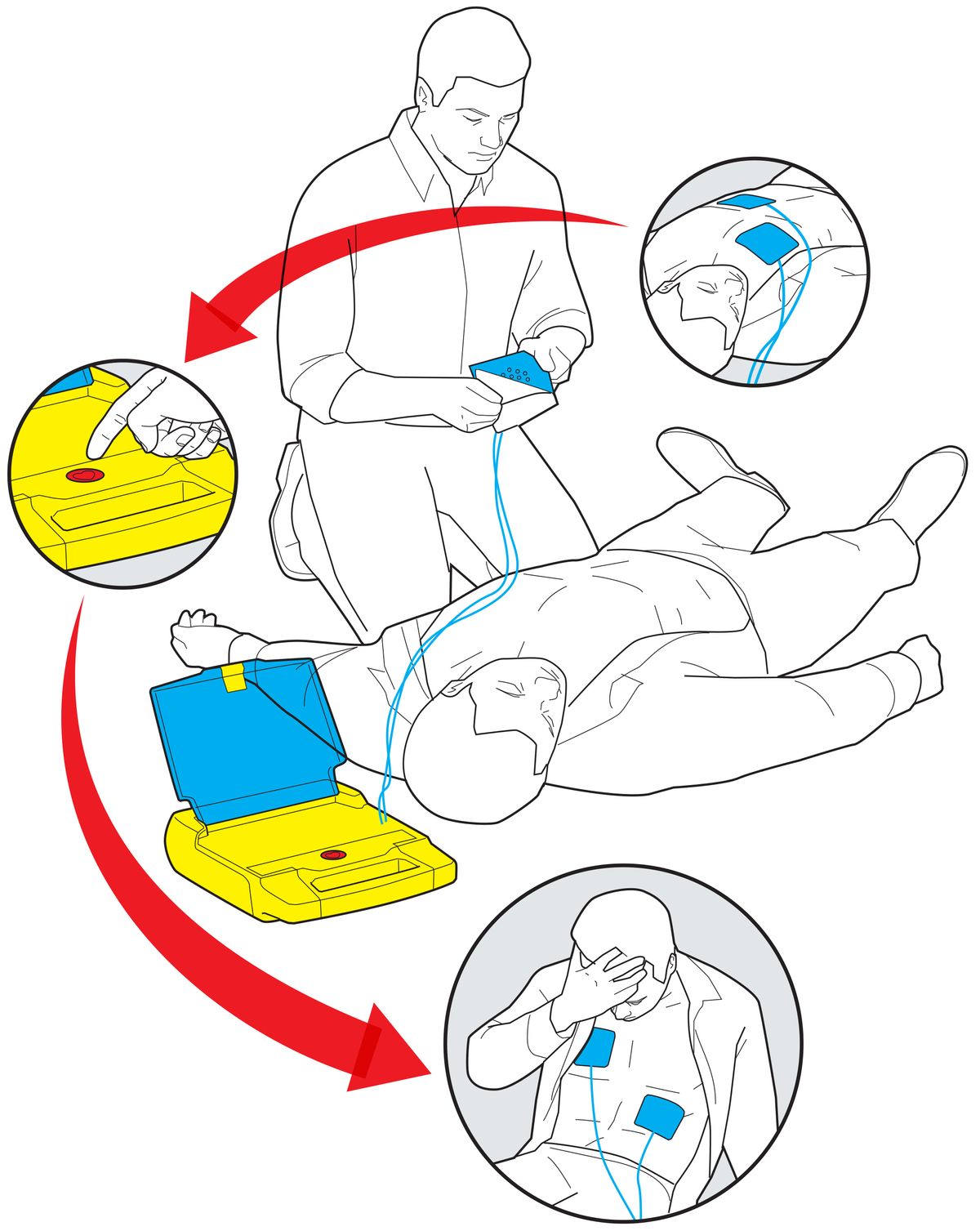
Luckily for your colleague, the rest of your actions couldn’t be more straightforward. From the moment you open the AED’s brightly colored box, audio instructions prompt you with simple commands. First, they tell you to place two adhesive electrode pads on your coworker’s bare chest, following the diagrams on the pad wrappers. The defibrillator’s built-in electrocardiograph automatically detects and analyzes the state of the patient’s heartbeat, and its software judges whether to administer a shock. Your colleague’s heart is indeed in ventricular fibrillation, so the AED asks you to approve the delivery of a tailored burst of electric current. “Push the button,” announces a firm voice emanating from the box. There’s only one button, so you press it. Your coworker’s body jumps a little, and that may be all that’s needed to restore a normal rhythm to his heart. If not, the box prompts you to push the button again after some time. Thanks to your quick actions, your colleague is saved.
Meanwhile, what’s happening inside the AED is a technical marvel. The device performs two main functions. First, it needs to recognize the lethal haywire rhythm of ventricular fibrillation. Second, it needs to deliver a 100-kilowatt shock to the heart. This jolt allows the heart to restart its normal rhythm, sort of like a Ctrl-Alt-Del for the organ. If the shock is delivered in the first minute of ventricular fibrillation, in more than 90 percent of cases the heart will regain a normal sequence of electric signals, and the steady contractions will return. It took decades of careful engineering to develop a device that could perform those two functions reliably, have a long shelf life, and be both safe and easy to use.
The inherent violence of an electric shock stands in stark contrast to its potential therapeutic effect. In fact, the origin of electrical defibrillation can be traced to both electrocution and efforts to electrify the United States in the early 1900s.
Engineers and medically inclined experimenters had long observed and tinkered with the potential of restoring life with electricity. Some of the first dedicated research into the mechanisms of electric shock, however, emerged from quite the opposite effect—electricity’s ability to kill. When General Electric, the company cofounded by Thomas Edison, switched from direct-current to alternating-current transmission in the early 1900s, linemen began to die from accidental electrocution. In response, GE funded research at several universities to study what made electric current lethal. Two electrical engineering professors, William Kouwenhoven and Guy Knickerbocker, at Johns Hopkins University, in Baltimore, tested the phenomenon by shocking stray dogs to death. Serendipitously, they noticed that a second ac shock could sometimes bring an electrocuted dog back to life.
Simply Saved: The automated external defibrillator, which shocks hearts out of cardiac arrest, hides its sophisticated engineering behind a simple and cheery exterior.
Kouwenhoven and Knickerbocker’s observation was picked up by a pioneering cardiac surgeon, Claude Beck, at the University Hospitals of Cleveland. He began delivering ac directly to the exposed hearts of animals he had put into ventricular fibrillation. Beck might have continued methodically with his animal experiments, except that in 1947 a 14-year-old patient’s heart stopped during surgery. Out of desperation, Beck ordered that his research unit be brought up from the hospital’s basement. This simple defibrillator consisted of a transformer to isolate the patient from the 110-volt ac wall supply, a variable resistor to limit the current to a heart-safe value, and two metal tablespoons with wooden handles to deliver the jolt to the exposed heart [see “Saved by a Spoon”].
The first shock failed, so Beck administered a second. That brought the patient back to life, and the event made national news. But because so little was known about why the technique worked or how to improve it, these crude ac systems persisted for several years. Recipients of closed-chest ac defibrillation tended to suffer unpleasant side effects from the large steady currents, including broken ribs and damage to the heart muscle—if they were saved at all.
Unknown to Beck and his colleagues in America, investigators in Europe and Russia were far ahead of them in animal research and were beginning to use a single pulse, or dc, defibrillation. In the 1890s, Jean-Louis Prévost and Frederic Batelli, two physiologists at the University of Geneva, revived animals with a capacitor discharge delivered directly to the heart.
Decades later, one of their graduate students, Lina Schtern, moved to the Soviet Union and continued to refine the technique—that is, until she received a death sentence during a crackdown on intellectuals under Joseph Stalin. She was eventually pardoned by the dictator himself, who (according to accepted rumor) believed that she could bring people back from the dead.
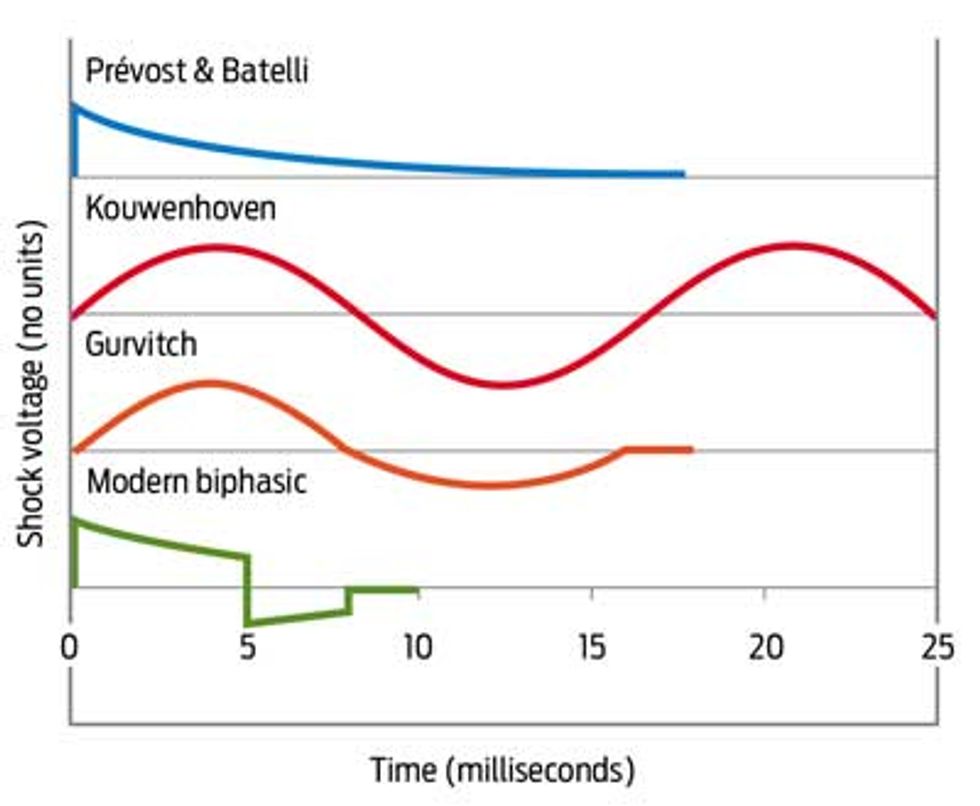
In Moscow, at the peak of World War II, a young student of Schtern’s named Naum Gurvitch made rapid advances in defibrillation. Gurvitch was the first to suggest using a biphasic waveform—a sizable positive jolt followed by a small negative pulse—to deliver the shock. That insight proved to be one of the most critical technical advances in the development of defibrillators. He published a short article on his use of biphasic shocks to resuscitate animals, but the idea didn’t really take hold until a Lithuanian-born American cardiologist, Bernard Lown, built a dc defibrillator. Lown used his defibrillator to treat patients suffering from ventricular tachycardia, a less ominous arrhythmia that also responds to shocks. He later had defibrillators built using Gurvitch’s schematics, but most U.S. researchers still refer to Gurvitch’s breakthrough discovery as the Lown waveform.
The core component of a defibrillator has always been the capacitor, which stores the energy needed for the shock. The first “portable” defibrillator, built in 1965, weighed 70 kilograms without batteries and was designed to be plugged into an ambulance’s starter battery. The device consisted of a large Mylar-film capacitor capable of storing a 4000-V, 400-joule charge, a charging circuit (to convert the 12 V from the car battery to 4000 V), a gas discharge relay, and a large inductor. When the capacitor discharged, the current passed through the inductor to reduce its peak current, which, at up to 80 amperes, might damage the heart instead of defibrillating it.
This early device had a button that controlled the gas discharge relay; when pressed, it completed a circuit and delivered a strong single pulse from the capacitor through the inductor to the patient. The current was transmitted through paddles the size of clothing irons placed on the chest so that the administrator could get a good grip. Because dry skin is an insulator, a rescuer had to apply sufficient pressure to overcome its impedance. (The Johns Hopkins engineers Kouwenhoven and Knickerbocker noticed that this heavy pressure also caused a patient’s blood pressure to spike. That’s how the chest-compression technique now used in cardiopulmonary resuscitation, or CPR, was invented. [See the online sidebar, "Rethinking CPR."])
These defibrillators required two trained operators: someone to press the paddles against the chest and someone else to push the button. One of the operators had to first acquire and interpret the electrocardiogram, or EKG. Then someone had to remove the leads from the EKG because the shock would otherwise destroy its electronics.
The device wasn’t truly portable—even after some design modifications, the cumbersome units weighed between 20 and 40 kg. There was also no safeguard to keep the device from being used in the wrong situation—say, erroneously shocking an unresponsive individual who might actually be experiencing a seizure or a fainting spell.
Two major advances cut the energy requirement for defibrillation in half, from 400 J to 200 J, while significantly reducing the failure rate. The first, which took hold in the 1980s, was to eliminate the bulky paddles.
Unlike the electrons that carry current through wires, current in the body is carried by ions, chiefly sodium, potassium, and chlorine. When the electric potential across a cardiac cell’s membrane reaches a certain threshold, ion channels open, allowing ions to enter the cell and trigger muscle contraction. In place of the defibrillator’s paddles, biomedical engineers developed flexible adhesive patches coated with a metal chloride gel (such as tin chloride) to transfer current from the wires to the body. Each patch has a different polarity: on the patch with a negative polarity, for example, an electron from the wire replaces a negative ion from the chloride in the gel, which frees the chloride ion to pass through the skin and carry current into the body.
The patches reduced the typical contact resistance from about 150 ohms to about 75 ohms, which allowed for smaller voltages. The lower voltages meant that defibrillators could be built with higher-density electrolytic capacitors and smaller semiconductor switches. Thanks to the adhesive patches, the defibrillation operation now required only one person.
However, these gelled patches sometimes dried out, so paramedics had to check their electrodes every day. Two of us (Karl Kroll and Byron Gilman) had the idea of packaging the two pads together with a partially conductive release liner between them. Now the AED was able to pass a small shock between the patches to prevent them from getting dried out.
The other big advance was to switch to the modern biphasic waveform, which, in addition to its higher efficacy, also reduces the power requirements for defibrillation [see “Wave Sculpting”]. One of us (Mark W. Kroll) was the first to publish a quantitative description of the biphasic waveform, in 1994. In essence, the first phase of the shock charges the cell membranes; the second phase, where the current reverses, returns the cell membranes to zero voltage. We still don’t know entirely why the biphasic waveform is so effective, and we have yet to learn of any analogues in the rest of biology. One purpose of the second phase appears to be to discharge and heal the blasted-open membranes of cells closest to the electrodes (which receive the most extreme current) and to discharge cells that are only marginally charged. Mark coined the term “burping” (based on the idea of a mother burping excess gas from an infant after feeding it) to describe this strange phenomenon.
Because biphasic waveforms require less power than their precursors, the size of a defibrillator’s components could also shrink. The heavy metal film capacitor was replaced by a lightweight bank of aluminum electrolytic capacitors connected in series, and the heavy iron inductor was eliminated altogether, as it was no longer needed to reduce the peak currents.
Wave Sculpting: From the 1890s, experimenters defibrillated with some success. Naum Gurvitch’s waveform, however, became the bridge to the modern AED.
With the accompanying reduction in peak voltages from 4000 V to less than 2000 V, the gas arc relay was dropped; switching is now performed by modern compact insulated-gate bipolar transistors. These are configured in a classic H-bridge circuit, the component that allows motor controllers to run forward or backward [see “Tender Loving Shock”]. Depending on which two of its four switches are closed, the circuit can deliver a normal or reversed-polarity voltage. Two switches are turned on for 5 to 8 milliseconds to deliver the main shock. Immediately afterward, the two remaining switches are turned on to deliver the residual capacitor charge and perform the burping function of the second phase.
Taken together, these changes reduced the weight of the unit from 40 kg to 1.5 kg and made it safer to operate. Further advances in the capacitor, battery, and high-voltage semiconductors should eventually reduce the size of the AED to that of a deck of playing cards.
The next challenge was to design the brains of the machine. The defibrillator had to figure out, on its own, when to deliver a shock.
The heartbeat is most vulnerable during a period known as the T wave. The T wave occurs just as the heart is beginning to relax after contraction and lasts about 100 ms, or about one-tenth of a heartbeat. A shock administered to a nonfibrillating heart during the T wave could potentially induce fibrillation. If the AED’s electrode patches are positioned far from the target, such as on the belly, the current that finally reaches the heart could be sufficient—if it arrives during that vulnerable period—to induce fibrillation, but it may not be strong enough to then defibrillate and undo its own damage. To provide a check on such situations, AED designs began incorporating an analysis system that checks for a pulse.
Recall that fallen colleague of yours. Once the AED is turned on and you’ve attached the electrode patches, the device’s first task is to recognize the EKG signal to see if ventricular fibrillation has occurred. The system starts by delivering a low-voltage, almost imperceptible 30-kilohertz signal through the two electrodes. That action measures the impedance to verify a good contact on the body.
When you get an EKG at the hospital or in a clinic, your right leg is used as the ground, or common, electrode from which to measure the tiny voltage differences that make up the EKG. However, as the astute engineer will notice, an AED has only two electrodes—there is no ground electrode. So the average voltage at these two electrodes is used as the reference.
The two-electrode signal is then fed into a very high common-mode-rejection amplifier, which differentiates between the two signals by rejecting the voltages common to both. (Additional complicated circuitry protects this microvolt-sensitive amplifier from the 2000-V shock—20 million times the EKG voltage—delivered to those same electrodes used for the sensing.) A sophisticated peak detector then analyzes the signal in search of a heartbeat. A normal heartbeat is essentially a cycle of blood-pressure increases and decreases, which show up on the cardiogram as clear voltage peaks followed by comparatively flat regions. In ventricular fibrillation, those distinct peaks disappear, and instead a noisy, messy signal will appear on the cardiogram. The peak detector interprets this noisy signal as a series of rapid, randomly spaced heartbeats. The AED makes its initial diagnostic decision by measuring the heart rate. If this rate is more than 150 beats per minute (2.75 hertz), the defibrillation-detection algorithm presumes that ventricular fibrillation has occurred, and the device will announce that the rescuer should administer a shock.
However, dozens of subtle issues can undermine this process. For example, if a patient has an internal pacemaker, that device’s higher-amplitude 60-beats-per-minute pulses could confuse the AED’s peak detector into ignoring the 100â¿¿microvolt ventricular fibrillation signal, so no shock would be administered. The opposite problem can occur if the patient has atrial fibrillation, a fairly benign rhythm disturbance in which the heart still perceivably beats but at irregular intervals. This condition can generate high heart rates, potentially causing the AED to call for an inappropriate shock.
To deal with these confounding rhythms and other interferences, the defibrillation-detection algorithm performs a simple spectral analysis. What follows is one example of such an algorithm; many competing approaches exist. If too much of the signal occurs at a higher frequency (30 to 100 Hz), then noise contamination, perhaps from an ac power line or some skeletal muscle contractions, is suspected and the algorithm will move away from diagnosing ventricular fibrillation. To handle the possibility of atrial fibrillation, the algorithm calculates the average derivative of the EKG voltage. If the average exceeds a critical threshold, that tends to rule out atrial fibrillation. The EKG of a heart in atrial fibrillation has a higher proportion of flat regions of zero voltage, and therefore a zero derivative.
Those tests and more are performed during a three-second window, leading to a tentative diagnosis. The process is then repeated to produce three diagnoses. Only if two or all three analyses indicate ventricular fibrillation will the shock be authorized.
The single-button design was another key improvement over earlier AEDs. An untrained operator who’s facing a panic-filled, life-or-death decision should not be confronted with multiple buttons and expected to quickly figure out which button turns the unit on and which one administers the shock. The breakthrough idea of one of us (Karl) was to have the device turn on automatically and start speaking when its lid is opened.
Every time an AED is used, whether or not a shock is delivered, it sows the seeds for its own improvement. Every EKG is stored in semiconductor memory for later downloading to a computer at the emergency-response headquarters, from which data can be sent back to the AED’s manufacturers. Researchers use this large database of diagnoses to develop and refine future algorithms. Linear methods (such as fast Fourier transforms) and nonlinear techniques (such as neural networks) may soon improve the detection of ventricular fibrillation. These sophisticated signal-processing techniques are being tuned up to make the correct diagnosis, even in the presence of electromagnetic interference, muscle noise, and unusual arrhythmias that might be plaguing the heart. Today’s algorithms may well be supplanted in a few years by more advanced ones that will barely resemble the method described here.
Thanks to the advances in the AED, the weak link in the chain is now CPR. The classic protocol is for a rescuer to administer manual chest compressions and mouth-to-mouth ventilations until someone brings an AED or an ambulance arrives. This keeps blood oxygenated and moving to forestall brain death. Surprisingly, recent studies have shown that the chest compressions also move some air through the lungs, at least for a few minutes after the onset of cardiac arrest. As a result, mouth-to-mouth breathing is now being dropped from those protocols.
However, even trained responders will tire after delivering chest compressions for more than a minute. Researchers at Minneapolis-based Galvani, a company founded by Mark W. Kroll and headed by Gilman, are now exploring an automated electrical form of CPR. Using the same defibrillation patches, this technique relies on complex, lower-voltage waveforms (100 to 200 V) that are delivered once or twice per second and cause strong chest constrictions. The constrictions appear to move blood as effectively as would chest compressions performed by a trained human rescuer.
If this research pans out, in the future a bystander need only attach the patches and the AED will do the rest, performing electrical CPR for a minute or two, followed by a calculated defibrillation shock if it’s needed. Indeed, we may now be on the cusp of a wave of medical automation that allows ordinary individuals to intervene constructively when other people’s lives are at stake. The AED, we think, serves as an important case study for how to fit sophisticated life-saving medical electronics into health care and rehabilitation outside of hospitals. Advances in portable and easy-to-use equipment, home-based therapy, remote health monitoring, and telemedicine may one day allow patients to avoid long, expensive, and emotionally draining hospital visits.
As their efficacy and ubiquity continue to grow, AEDs, we hope, will pave the way for a future where emergency health care is available to all.