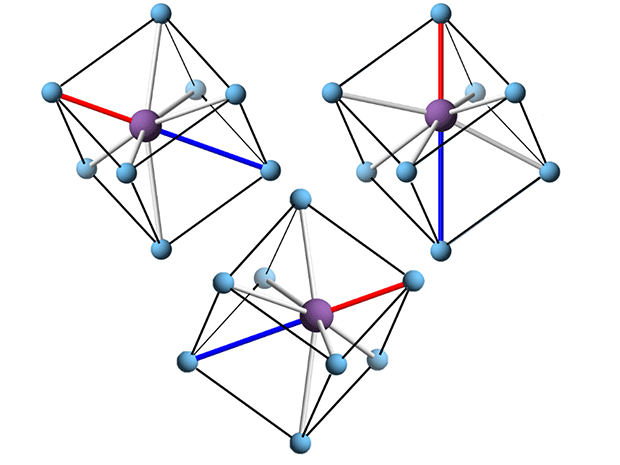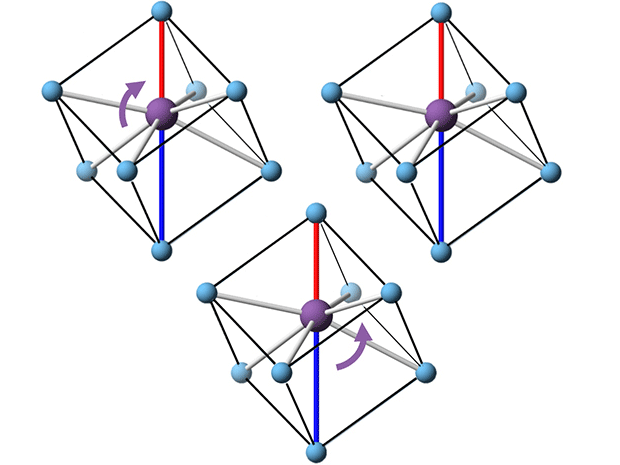
Atoms within the tiny crystals in many dielectric and ferroelectric ceramics twist and dance when an electric field is turned on. But until now, nobody could tell exactly how they twisted and danced, even though understanding those movements could be the key to developing more compact, higher performance capacitors and condensers.
In a new Scientific Reports paper, researchers at North Carolina State University—with colleagues from the U.S. National Institute of Standards and Technology and the University of New South Wales—show how new ways of analyzing X-ray diffraction data can reveal the details in a ceramic’s structural response to an electric field.
A ceramic can be a complex jumble of amorphous material that glues together crystalline particles of different sizes in different orientations. Traditionally, scientists probe crystal structures by reading the interference patterns produced by X-rays passing through them. They usually analyze only the primary signal, the strongest bands or brightest rings, which give information on the overall structure. This works well on homogeneous material in regular lattices. In a heterogeneous ceramic, though, with crystals pointing every which way, a lot of detail is buried in the faint secondary signals and diffusion patterns.
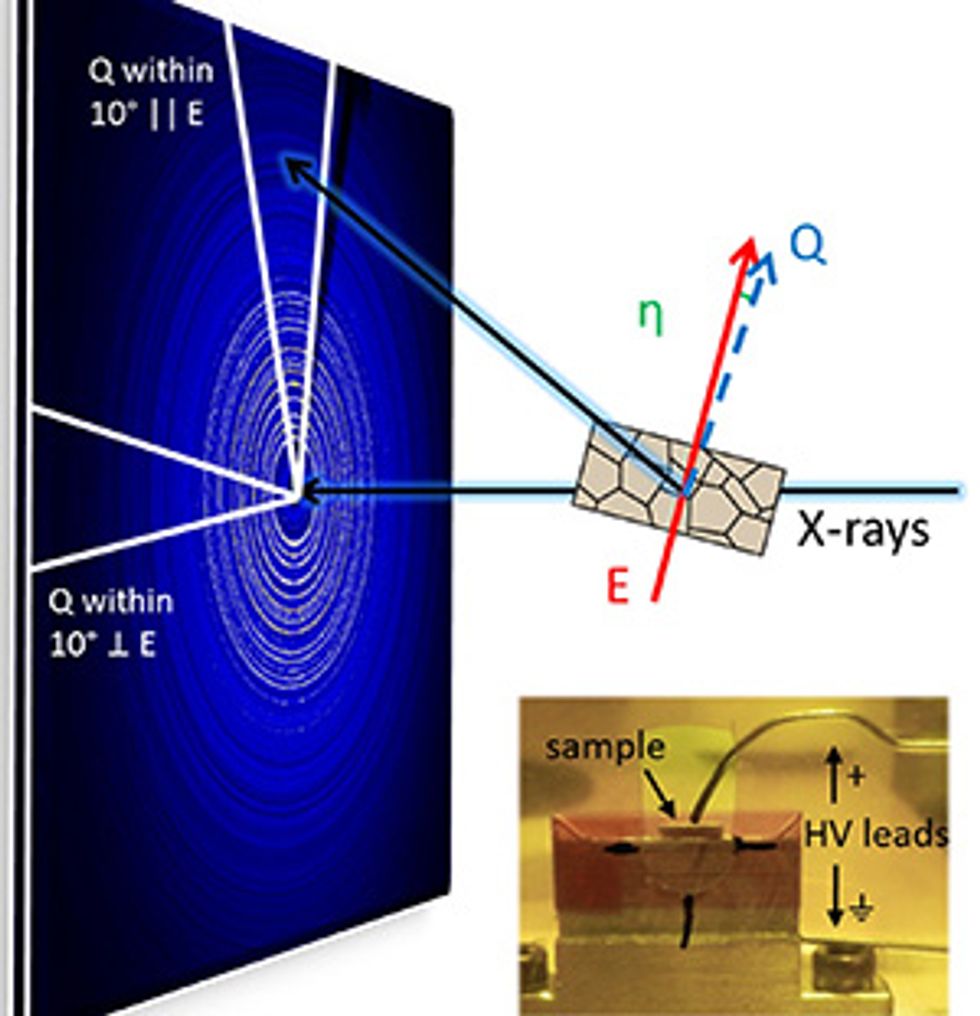
The NCSU-led team captured this detail by applying the pair distribution function (PDF). The method, first described in the 1960s but used increasingly over the past decade, lets researchers read all of the signals in a wedge of the diffraction pattern to calculate the length and orientation of the bonds between pairs of atoms. (See diagram.) Researchers take snapshots of the ceramic with and without an applied electric field, taking a census of how many microcrystals are in which orientations in each condition.
"A good analogy would be that analyzing the bright rings is like examining a skyscraper from far away and determining that each office is 500 square feet,” said NCSU’s Tedi-Marie Usher in a press release. “However, by also analyzing the weak X-rays scattered from the sample, we can determine that some offices are 400 square feet and others are 600 square feet, and some have the desk on the east side, and others have the desk on the north side."
The research team analyzed three perovskite materials— barium titanate (BaTiO3), sodium bismuth titanate (Na0.5Bi0.5TiO3), and strontium titanate (SrTiO3)—all members of a class of ceramics with useful dielectric, ferroelectric, and piezoelectric properties.
In an electric field, dielectrics become polarized and ferroelectrics reverse their polarity. This segregation—positive charge on one side, negative on the other, nobody in the middle—impedes current flow across the gap, letting energy build up. The material’s relative permittivity (or dielectric constant) is an index of how effectively it can store energy as an electric field. Air and a vacuum have permittivities of 1 (or very close to it). Silicon’s is 12. In barium titanate (which is also the first piezoelectric ceramic identified), the permittivity can range up from 1,200 to 10,000 or more.
As the material polarizes, its atoms reorient themselves in their crystal lattices. In sodium bismuth titanate, for example, bismuth atoms align with the electric field, changing their relationships with the surrounding titanium ions. (See animation. The bismuth atoms are shown in purple, the titanium in blue.)
“Dipolar effects are known to have large contributions to dielectric permittivity,” said NCSU’s Jacob Jones, the team leader, in a press release. “The measurement tells us the population of dipoles that are reorienting. We could combine this information with the microscopic spontaneous dipole magnitude and calculate a net contribution to the macroscopic dielectric permittivity.”
Right now, there appears to be no one-size-fits-all mechanism for structural shifts in dielectric ceramics.
“One of the interesting findings here is that each of the three dielectric materials we tested exhibited very different behaviors at the atomic level,” Jones said. “There was no single atomic behavior that accounted for dielectric properties across the materials."
So there is much more to be learned. Said Jones:
Of immediate interest are a broad range of dielectric materials that exhibit complicated (and elusive) structures. For example, perovskites with components Na0.5 Bi0.5 TiO3 [sodium bismuth titanate], K0.5Bi0.5TiO3 [potassium bismuth titanate], and Pb(Mg0.33Nb0.67)O3 [lead magnesium niobate] and their solid solutions. These compounds exhibit unique local structures that will respond to field differently. This is a useful technique to reveal interesting physical origins of unique dielectric, piezoelectric, and ferroelectric properties of these materials. We are also interested in extending this technique to an emerging class of high entropy alloys (HEAs) and entropy-stabilized oxides (ESOs), where unique elements may behave differently in response to mechanical stress or electric fields. The unique local behavior of the elements in these multi-element materials is not accessible in traditional diffraction measurements.
Douglas McCormick is a freelance science writer and recovering entrepreneur. He has been chief editor of Nature Biotechnology, Pharmaceutical Technology, and Biotechniques.