It’s Big and Long-Lived, and It Won’t Catch Fire: The Vanadium Redox-Flow Battery
Move over, lithium ion: Vanadium flow batteries finally become competitive for grid-scale energy storage

The factory sprawls over an area larger than 20 soccer fields. Inside, it’s brightly lit and filled with humming machinery, a mammoth futuristic manufactory. Robot arms grab components from bins and place each part with precision, while conveyor belts move the assembled pieces smoothly down production lines. Finished products enter testing stations for quality checks before being packed for shipping.
It has been called a gigafactory, and it does indeed produce vast quantities of advanced batteries. But this gigafactory is in China, not Nevada. It doesn’t make batteries for cars, and it’s not part of the Elon Musk empire.
Opened in early 2017, in the northern Chinese port city of Dalian, this plant is owned by Rongke Power and is turning out battery systems for some of the world’s largest energy storage installations. It’s on target to produce 300 megawatts’ worth of batteries by the end of this year, eventually ramping up to 3 gigawatts per year.
The scale of this “other” gigafactory may be impressive, but the core technology it makes is even more compelling. The Dalian factory produces vanadium redox-flow batteries, a specialized type whose time has finally come. The VRFB was invented decades ago but has emerged only recently as one of the leading contenders for large-scale energy storage.
How large? VRFBs are being touted for grid-scale uses in which they would store up to hundreds of megawatt-hours of energy. In these applications, they may be charged by large baseload power plants, which generate electricity cheaply but are too sluggish to accommodate sharp increases in demand during peak hours. Or they may be charged by renewable sources like wind farms, whose generation doesn’t always align well with demand. Like most batteries, VRFBs can deliver power nearly instantaneously, so they can stand in for the traditional means of meeting peak demand: fossil-fueled “peaker” plants that, in comparison with batteries, are costly to maintain and operate and not as fast.
Lithium-ion batteries, too, have been proposed for grid-scale uses. But here they are no match for VRFBs, which have longer lifetimes, can be scaled up more easily, and can operate day in, day out, with no significant performance loss for 20 years or more.
Soon this technology will be the cornerstone of the largest battery installation in the world: a 200-MW, 800-megawatt-hour storage station being built in Dalian. The first 100 MW will be installed by the end of this year, with the remainder coming on line in 2018. The station will help balance supply and demand on the Liaoning province power grid, which serves about 40 million people, filling the same function as a peaker power plant but without using scarce water. Furthermore, if the batteries are charged by the wind-generated power that’s abundant in northern China, no fossil fuels will be burned. Should demand spike or the supply dip suddenly, the battery station will be able to dispatch all or just part of its 200 MW within milliseconds.
The result will be a stable grid that can integrate more renewable energy. At times, wind generation in Liaoning province tops 7 GW, or about 15 percent of total generation. But much of that power isn’t used because other sources already meet grid demand. Earlier this year, the amount of wind power in Liaoning that was curtailed, or wasted, reached 15 percent; in the neighboring province of Jilin, it was 30 percent. The Dalian site will store that wasted energy for later use, adding up to a few hundred gigawatt-hours per month.
The Dalian site is just one of several big VRFB installations being built in China, so its reign as the world’s biggest battery may be short. Meanwhile, other countries are adopting VRFBs. According to the U.S. Department of Energy’s global energy storage database, since 2014, more than 30 VRFB projects in 11 countries have been deployed or begun construction; these range in power from a few tens of kilowatts up to Dalian’s 200 MW. While these projects reflect the surging interest in all forms of energy storage, what’s driving the renewed push toward VRFBs are important technological distinctions.
Today’s state-of-the-art vanadium redox-flow batteries started out as a modest research project at the Pacific Northwest National Laboratory (PNNL), a U.S. Department of Energy lab in Washington state. The PNNL team, which I led, came together in 2007, at a time when world oil prices were steadily climbing. The economies of China and India were experiencing double-digit growth, and environmentalists were concerned about the accelerating rate at which they (and other countries) were consuming fossil fuels. In the United States, awareness was starting to build about the potential of renewable but intermittent energy sources like wind and solar.
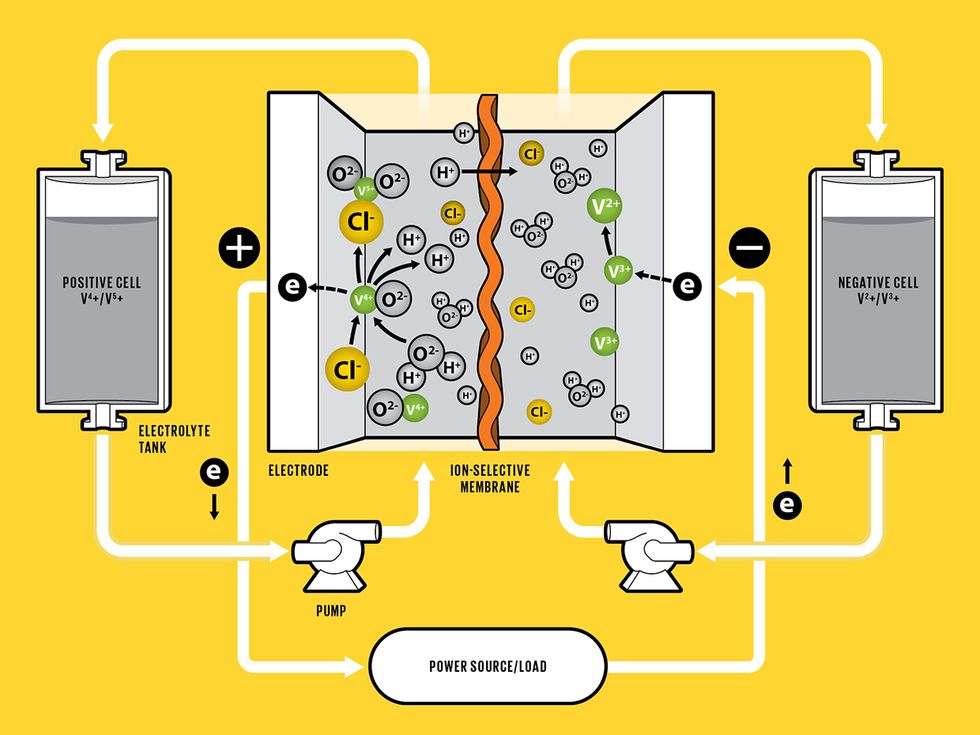
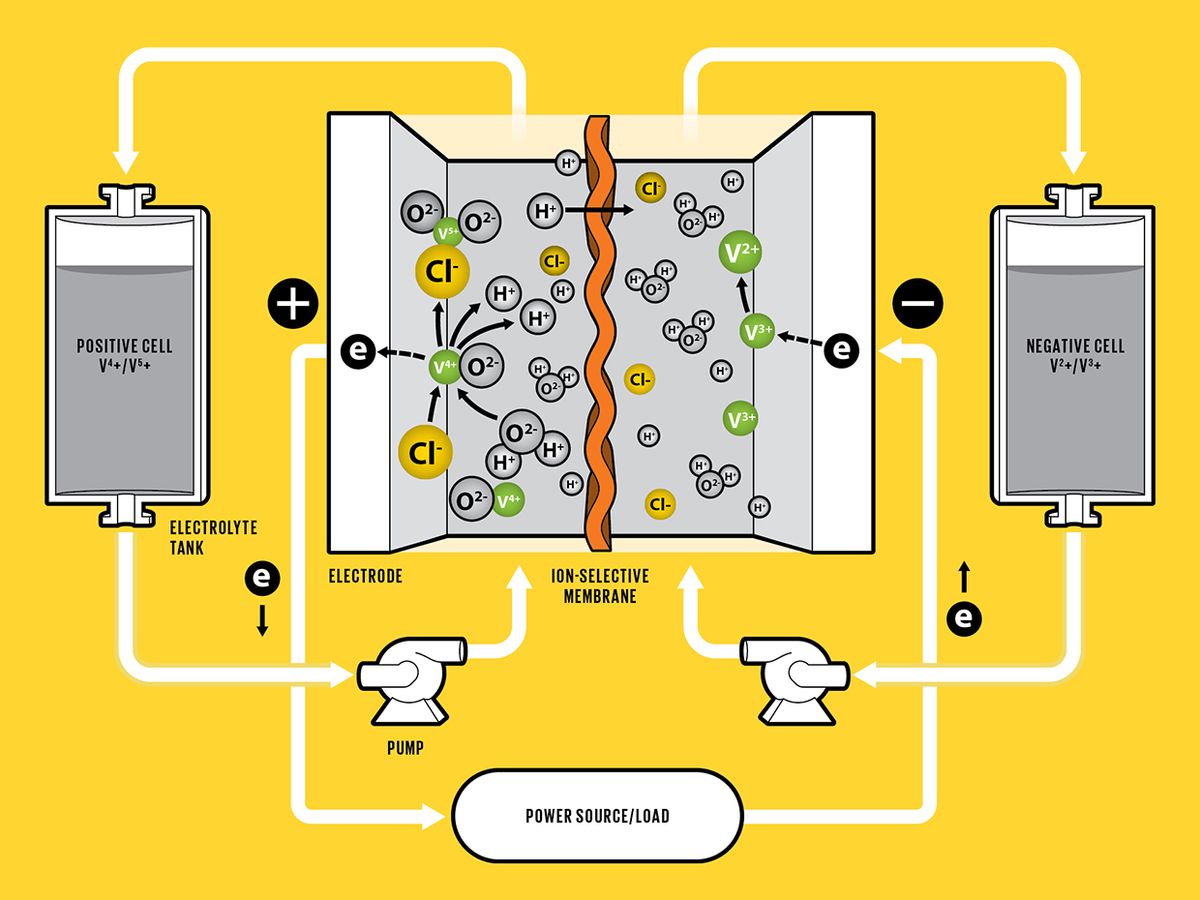
This Battery Flows
The positive and negative sides of a vanadium redox-flow battery are separated by a membrane that selectively allows protons to go through. During charging, an applied voltage causes vanadium ions to each lose an electron on the positive side. The freed electrons flow through the outside circuit to the negative side, where they are stored. During discharging, the stored electrons are released, flowing back through the outside circuit to the positive side.
Against that backdrop, we decided to search for a better way to store renewable energy as a means of promoting its adoption while also improving grid reliability. Our group included the lab’s top experts on power, materials, and chemistry, as well as an intellectual-property lawyer, Peter Christiansen, who has a background in power engineering. Peter helped focus our efforts on technologies that would have the greatest societal impact. In 2009, our group began receiving significant support from the DOE’s Energy Storage Program, which boosted our annual R&D budget to US $10 million.
At the outset, it wasn’t at all obvious that flow batteries were the way to go. Indeed, we started off by reviewing all of the various battery technologies, including lithium ion, sodium sulfur, advanced lead acid, redox flow, and a few other novel concepts.
Then as now, attention was being lavished mainly on high-power lithium-ion batteries. According to Greentech Media, over 90 percent of energy storage systems deployed in the United States in 2015 and 2016 were Li-ion batteries, and that’s likely to be true this year as well. The technology has steadily advanced as a result of several decades of intense work on batteries for electric vehicles and also mobile devices, such as laptops, tablets, and smartphones. Tesla’s Powerwall, for example, is intended for residential and business energy storage, but it uses essentially the same Li-ion cells as those in the company’s electric cars.
For the grid-scale applications we were targeting, however, lithium ion has major shortcomings. To make battery packs capable of storing megawatt-hours requires many thousands or even millions of Li-ion cells, each of which needs to be managed individually as well as collectively with the other cells. Even then, Li-ion batteries can supply power over only relatively short durations, typically 2 hours or less.

To create a longer-duration battery, manufacturers would have to make the electrodes thicker and pack them with more active materials, which would raise the price. Or utilities could simply install more Li-ion battery packs, adding to the cost.
The performance of a Li-ion battery also degrades over time, giving the battery a typical lifetime of 10 years or so. That may be fine for a family car but is less desirable for use on the power grid. And Li-ion batteries have known safety issues, most notably the occasional explosion or spontaneous fire.
We also looked at sodium-sulfur batteries. Once considered quite promising for utility-scale installations, this type of battery operates at around 300 °C to liquefy the sulfur and sodium, which respectively form the positive and negative electrodes. But these batteries, too, have flammability issues. After a 2-MW system at a plant in Joso City, Japan, caught fire in 2011, for example, the battery maker, NGK Insulators, recalled its products and temporarily halted production.
Redox-flow batteries (RFBs), by contrast, offer features not found in other batteries. In theory, they can be easily scaled up to megawatt-hours, sustain their performance over much longer lifetimes, and be much safer, if built around nonflammable materials. In contrast to Li-ion and other solid-state batteries, which store electricity or charge in electrodes made from active solid materials, an RFB works more like a reversible fuel cell: To discharge, the battery takes the chemical energy stored in liquid electrolytes and converts it into electrical current, reversing the process to charge.
Each flow battery cell has a negative side and a positive side. Depending on whether the battery is charging or discharging, each side will either produce or take in electrons that are flowing through an outside circuit. The two sides are separated by a membrane that selectively allows protons to pass through. During charging, a voltage applied across the positive and negative sides causes vanadium ions in the electrolyte—which is flowing through the battery’s stack of thin, platelike cells—to each lose an electron on the positive side. The freed electrons pass through the outside circuit to reach the negative side, storing electrical energy. During discharging, the accumulated electrons on the negative side flow back through the outside circuit and are taken up on the positive side, releasing the stored electrical energy.

The first redox-flow batteries were developed in the early 1970s by Lawrence Thaller and his group at NASA, as a possible energy source for deep-space missions. They used an iron solution on the positive side and a chromium solution on the negative side. But the use of different elements led to cross-contamination, as iron and chromium ions tended to diffuse across the membrane separating the two solutions.
In the mid-1980s, Maria Skyllas-Kazacos and her group at the University of New South Wales, in Sydney, demonstrated an improved RFB that used the same element—vanadium—on both sides of the battery, so there was no risk of contamination. Vanadium is an abundant silvery-gray metal, cousin to niobium and tantalum, that is primarily mined in China, Russia, South Africa, and Brazil. But the early VRFBs couldn’t store much energy—just 12 to 15 watt-hours per liter of electrolyte. To serve any useful function, the batteries would have to be huge: A 1-MW/4-MWh system would have occupied an area equal to one or two basketball courts.
Another problem was that the vanadium oxide tended to precipitate out from the electrolytes, resulting in a gradual loss in the battery’s capacity. And as if those problems weren’t enough, keeping the vanadium in solution meant that the batteries could operate only within a narrow temperature range, between about 10 to 40 °C. The addition of thermal-management equipment and other control electronics further cut the battery’s overall efficiency while increasing its size and complexity.
These shortcomings notwithstanding, the PNNL team believed the technology had great potential. So we resolved to develop new electrolyte chemistry, membranes, and prototypes. With the VRFB’s inventor, Skyllas-Kazacos, serving as an advisor, we began by running simulations to investigate different electrolytes. We tested the most promising ones in the lab and optimized their chemistry.
Finally, in 2011, we succeeded in developing a new vanadium-based electrolyte that relied on reactions with a chloride solution. This seemingly simple change effectively doubled the energy density over that of existing VRFBs: Because more vanadium ions remained stable in solution, more of them were available during charging and discharging.

The resulting battery had a footprint that was one-third to one-fifth as large as its predecessors and could function within a much wider ambient temperature range, from 0 to +50 °C, without additional thermal management. Other modifications made the battery more reliable, simpler to manage, and more tolerant of electrolyte impurities.
PNNL licensed the breakthrough electrolyte chemistry to three U.S. companies, including UniEnergy Technologies (UET), a company that I cofounded in 2012 to commercialize the new VRFBs. In the five years since then, UET, based in Mukiteo, Wash., has successfully scaled up the batteries and deployed several megawatt-level field demonstrations. Our battery systems fit compactly in shipping containers, making them easy to transport and deploy.
We’ve also brought down the batteries’ cost: A few years ago, the cost of a 4-hour VRFB system was about $800 per kilowatt-hour. These days, it’s about half that, comparable to the cost of a stationary lithium-ion system. But that’s not an apples-to-apples comparison. As mentioned earlier, like that of other solid-state batteries, lithium ion’s capacity degrades over time, and its life span is shorter. We’ve tested individual VRFBs through more than 14,000 cycles, fully charging and discharging each cycle, and they still perform at 100 percent capacity. This should translate into a life span of 20 years or more. To date, our company has installed several megawatt-scale systems around the world, with an additional 200 MWh either awarded or in contract.
And there’s every reason to expect more utilities to opt for this energy storage technology. China looks set to lead the way. The country’s 13th Five-Year Plan for Power-Sector Development, released last year, aims to revitalize the electrical infrastructure to allow the integration of over 300 GW of wind and solar power by 2020. It cites energy storage as a key technology in realizing this ambitious goal and specifically recommends the scaling up of vanadium redox-flow batteries.
Meanwhile, in California, Hawaii, Massachusetts, New York, and other states, mandates and policies are colliding with simple economics to compel utilities to deploy energy storage at rates unimaginable even a decade ago.
As electricity customers come to expect every step of the electrical power chain—from energy generation through transmission and distribution to the end user—to be clean, efficient, reliable, flexible, and affordable, the case for energy storage grows ever stronger.
This article appears in the November 2017 print issue as “Is This The Ultimate Grid Battery?”
About the Author
Z. Gary Yang is cofounder and CEO of UniEnergy Technologies, in Mukilteo, Wash.