Nanoparticles have been suggested as a way to kill cancer cells in a multitude of ways. Recent research has suggested a method for surrounding gold nanoparticles with nanobubbles that would rip open small pores in cancer cell membranes. This would allow drugs present outside the cells to get in. Another cancer killing treatment is tricking lymphoma cells into eating gold nanoparticles. Once ingested, the nanoparticles make it impossible for the cancer cells to eat anything else, dooming them to death by starvation.
You may have noticed the recurring use of gold nanoparticles in cancer research. Following that tradition, researchers at ETH Zurich in Switzerland have demonstrated that gold nanoparticles, in combination with near-infrared light, can turn up the heat on cancer cells enough to kill tumors.
While gold nanoparticles are well tolerated by the human body, they are not too good at absorbing long-wavelength red light, which is able to penetrate human tissue better than short-wavelength blue light. The nanoparticles that are effective at this are known as plasmonic nanoparticles. Plasmonics is a field in which free electrons in a metal can be excited by the electric component of light so that there are collective oscillations in the material with heat generation being one effect.
The ETH Zurich researchers knew that if they molded the gold nanoparticles into a particular shape, such as a rod or a shell, they could give it the plasmonic property for absorbing near-infrared light it otherwise lacked. The problem with this approach is that is complex and expensive.
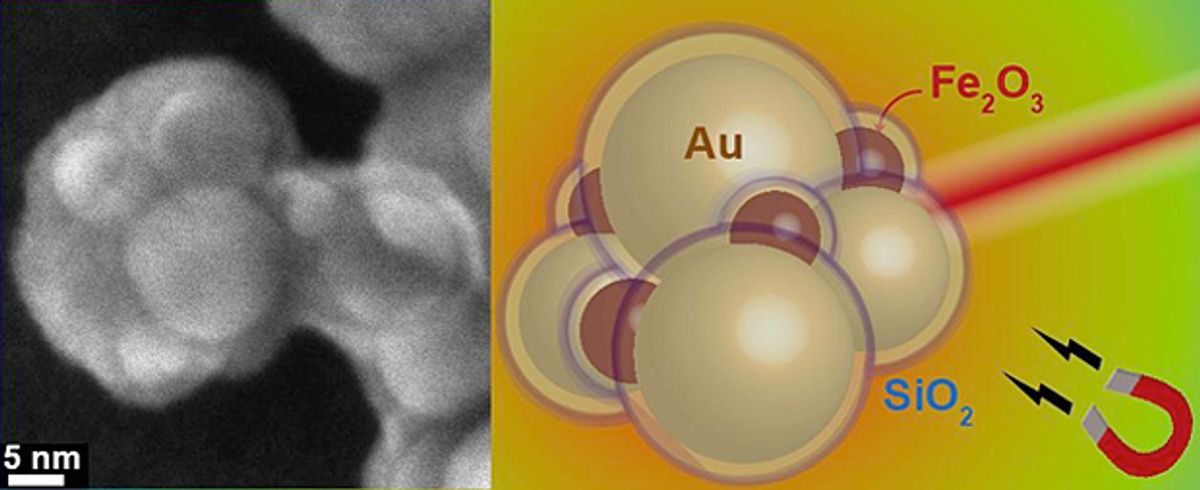
In research published the journal Advanced Functional Materials ("Photothermal Killing of Cancer Cells by the Controlled Plasmonic Coupling of Silica-Coated Au/Fe2O3 Nanoaggregates"), the Swiss researchers devised a way to make sphere-shaped gold nanoparticles aggregate into a light-absorbing design. To do this, each particle was coated with a silicon dioxide layer that serves as a buffer between the individual spheres in the aggregate. Maintaining precise spacing between each nanoparticle makes the configuration absorb the near-infrared light.
“The silicon dioxide shell has another advantage”, explains Georgios Sotiriou, lead author on the study, in a press release. “It prevents the particles from deforming when they heat up.” This is a critical feature since the particles' near-infrared light absorbing qualities are dependent upon them maintaining their spherical shape.
That’s great, but how do they reach the cancer cells so they kill the tumor when they heat up? The trick the researchers developed was adding superparamagnetic iron oxide particles in with the gold particles; the iron makes the nanoparticles steerable via magnetic fields so that they accumulate next to the cancer cells.
While issues still need to be addressed such as how the particles are removed from the body, the nanoparticles do lend themselves to being used as a contrast agent in magnetic resonance imaging.
“You could even couple the particles with temperature-responsive drug carriers, which would then allow the drug release if a certain temperature were exceeded,” explains Sotiriou in the release.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.