Biofuels, once hailed as a planetary savior and alternative to oil and gas, have not quite fulfilled that destiny. Traditional, mass-produced biofuels from crops such as corn carry a litany of problems, including land use issues and questions of life cycle emissions. If we could generate usable fuels from more benign sources, it could go a long way toward solving a host of energy and environmental problems. A team at Stanford University reports today in Nature that they have a novel way to produce ethanol from carbon monoxide (CO) gas using a metal catalyst made of copper nanocrystals.
"We have discovered the first metal catalyst that can produce appreciable amounts of ethanol from carbon monoxide at room temperature and pressure—a notoriously difficult electrochemical reaction," said senior study author and Stanford chemistry professor Matthew Kanan in a press release.
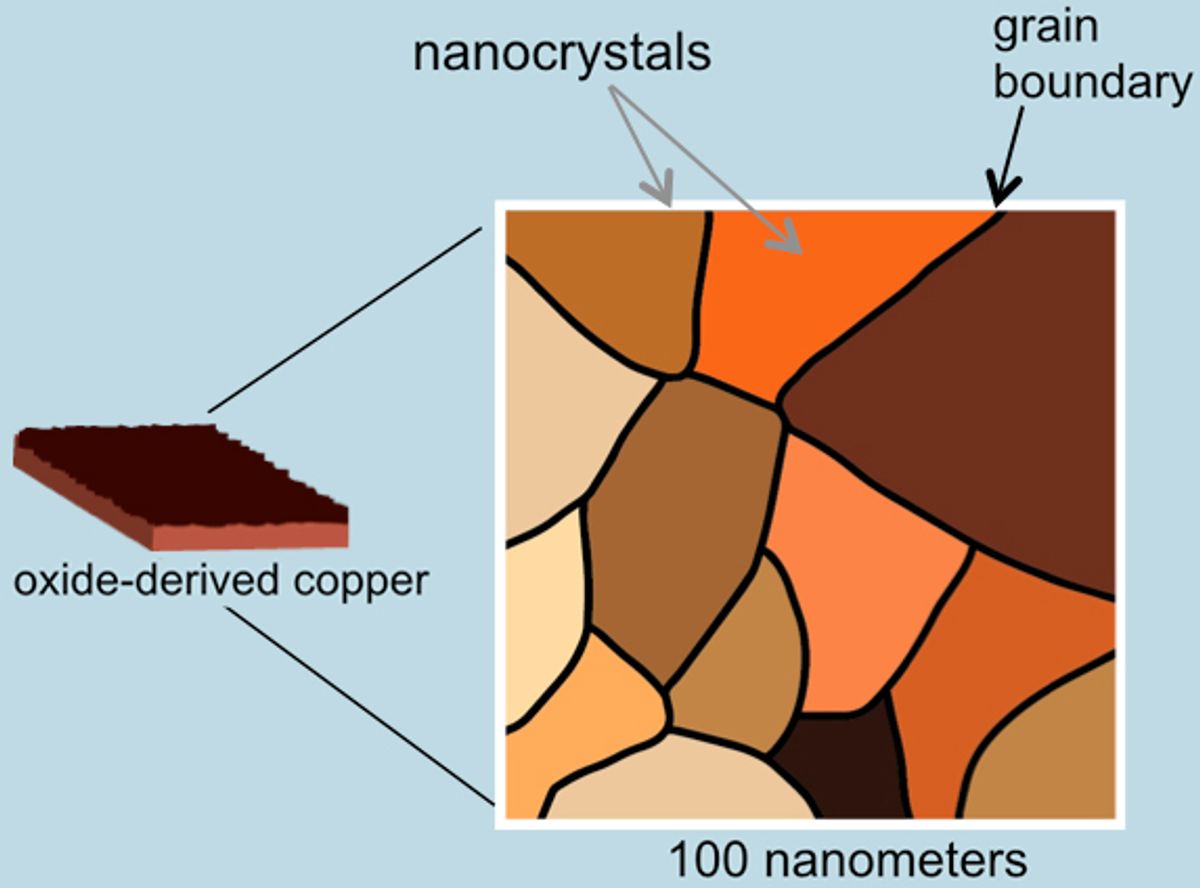
Copper is the only material known to electroreduce CO down to generate fuels, but it does so at extremely low efficiencies. Kanan's group improved this with a nanocrystalline form of copper produced from copper oxide; this new material improves the efficiency of the reactions dramatically.
The researchers built a fuel cell, including a cathode made of the new copper nanocrystals, and suspended it in CO-saturated water; a small voltage applied across the fuel cell generates the resulting ethanol products. The Faraday efficiency using the oxide-derived material was 57 percent, meaning more than half of the current used went toward producing ethanol and acetate. Standard copper particles, meanwhile, produced hydrogen almost exclusively (Faraday efficiency of 96 percent) and very little ethanol.
In an e-mail, Kanan said a few years is probably enough to turn this basic work into prototype devices, outside of the lab, that can produce meaningful amounts of fuel. "Some of the technical issues include reformulating the catalyst such that it can be dispersed on high-surface area electrodes, and engineering an electrochemical cell that delivers CO to the catalyst at a high rate," he said. The long-term stability of the oxide-derived copper catalyst is still in question as well. Kanan declined to offer guesses on eventual costs of the device, given its still lab-bound status; copper, however, is not particularly expensive, as catalysts go.
If the details of this were actually to work out and at an acceptable cost, it could be enormous. Though there have been changes proposed recently in the biofuels mandate in the United States, we're still producing billions of gallons of the stuff each year, virtually all of it from corn. The big idea with the new catalyst would be to power the fuel cell using renewable energy rather than from fossil fuels; ideally, one would just grab carbon dioxide out of the atmosphere and turn it into CO.
"There is good technology for converting CO2 to CO using an electrical energy input, although it requires high temperatures," Kanan told me. "There has been much work by many groups including ours to develop a low temperature electrochemical process for converting CO2 to CO, and a number of good catalysts have been found recently. I don't think the CO2-to-CO would be a limiting factor."
Dave Levitan is the science writer for FactCheck.org, where he investigates the false and misleading claims about science that U.S. politicians occasionally make.