Lithium-ion batteries are everywhere, powering phones, cars, and avionics, among other things. However, lithium is a relatively rare element, found in some locations in South America. That not only keeps the price of lithium-ion batteries high, but also makes the supply chain vulnerable to political instabilities.
Sodium has a very similar chemistry to lithium, and as soon as lithium-ion batteries came to market, researchers started looking to sodium as a substitute for lithium in rechargeable batteries. Unlike lithium, the reserves of sodium are practically unlimited. The highest hurdle for sodium to clear on its way to battery dominance is the development of suitable electrodes.
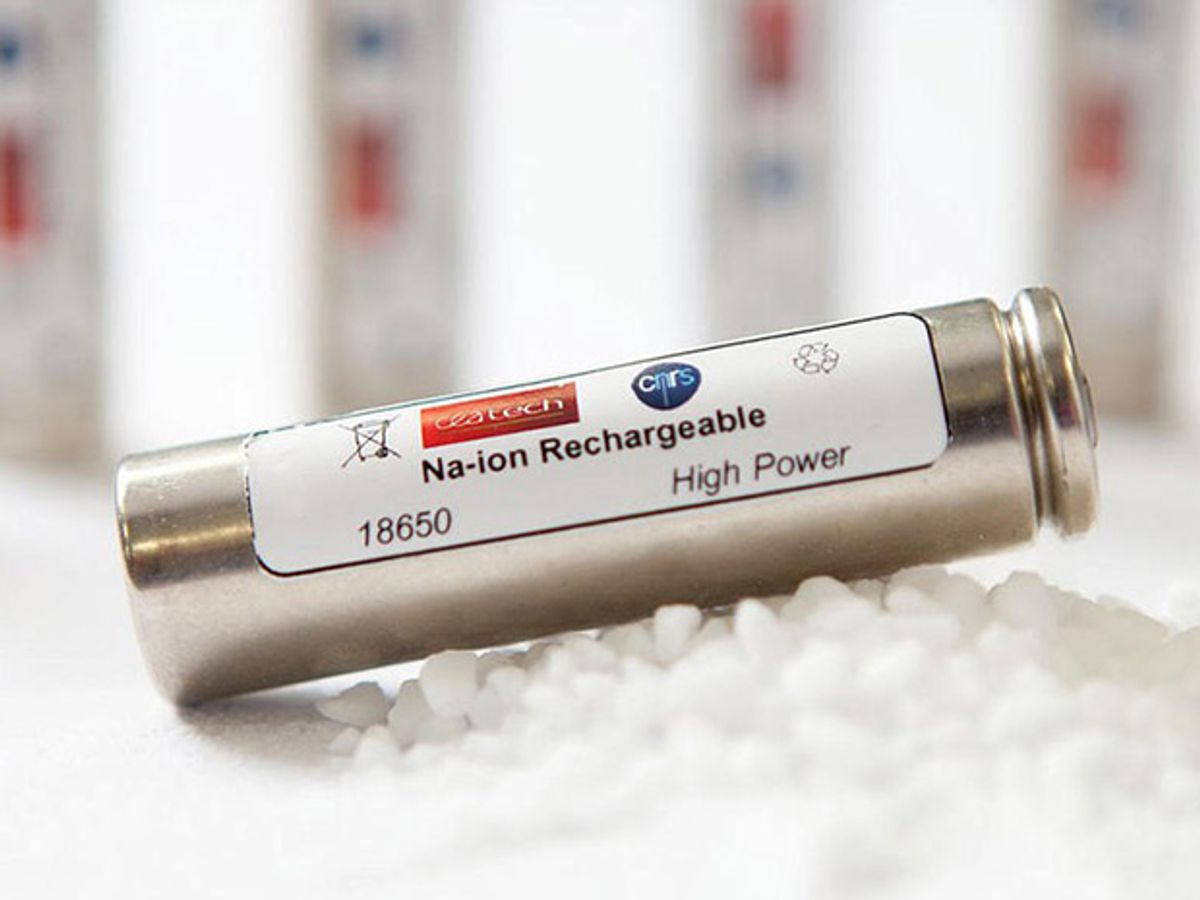
At the end of November a team of French researchers from the CNRS, the French National Centre for Scientific Research and the CEA, France's Alternative Energies and Atomic Energy Commission, announced in a press release and a CNRS News article that they had produced, in collaboration with the Research Network on Electrochemical Energy Storage, RS2E, a prototype sodium-ion battery that can store an acceptable amount of electricity in the same standard industry format as lithium ion batteries. It is slightly larger than an AA battery—18 mm x 65 mm.
Although the news article calls the composition of the negative electrode "a trade secret," the team applied for a patent in October of this year, describing a negative electrode with a layered structure based on the titanium oxide compound Na2Ti3O7, and a method to produce it.
Such electrode structures can store lithium ions, and recently researchers have begun investigating their suitability for storing sodium ions.
"The chemistry is very close to that of the lithium battery, and from that point of view there are no major difficulties; the mechanisms are the same ones and all the industrial processes for their production are the same," says Laurence Croguennec, a material scientist at the CNRS Institut de Chimie de la Matière Condensée de Bordeaux.
One remaining problem is that sodium is less efficient as a charge carrier. "A sodium battery loses 0.3 volts as to compared to a lithium battery. You have to develop materials that can function at higher voltages and that provide ample capacity," says Croguennec.
Whether sodium-ion batteries will reach parity with lithium-ion batteries is still an open question. "The development of the electrode has taken six months, so this gives us hope for improvement. The performance that has been announced is quite good for a starting point, and materials can be further optimized," says Croguennec. For the moment the sodium batteries have a storage capacity of 90Wh/kg, which is comparable to the early lithium batteries.
"We are not yet close to the energy levels that you can find, for example, in Tesla cars," says Croguennec. At this point, the most reasonable application for sodium batteries is renewable energy storage, as they could be cheaper per stored unit of energy and are scalable in size like lithium batteries, explains Croguennec.
The prototypes are not yet ready for the market, but CEA believes they will be of interest to industry. “We are now in discussion with possible industrial partners, says Croguennec.