Less Fire, More Power: The Secret to Safer Lithium-Ion Batteries
Curbing needlelike dendrites that short out cells will make batteries less likely to go up in flames

Lithium-ion batteries have made headlines for the wrong reason: as a fire hazard. Just this past May, three apparent battery fires in Tesla cars were reported in the United States and Switzerland. In the United States alone, a fire in a lithium-ion battery grounds a flight every 10 days on average, according to the Federal Aviation Administration. And the same problem afflicts electronic cigarettes, which have been blowing up in people’s faces sporadically.
No other drawback has so hobbled the advance of what is by far the most promising battery technology to emerge in our lifetimes. Lithium-ion batteries store much more energy than previous chemistries could manage, making them crucial to the future success of phones, drones, cars, even airplanes.
Solving this problem would not only protect lives and property, it would also make it possible to use larger battery packs with more closely packed cells. We’d finally be able to fully exploit the great energy-to-weight ratio, or specific energy, that this technology allows. What’s more, we’d be able to make progress with the next generation of batteries, the ones that use lithium metal.
The problems of today’s lithium-ion batteries can be traced largely to dendrites, tiny threadlike structures that form on the surface of an electrode over repeated cycles of charging and discharging. But through our work at Dartmouth and Stanford, the two of us have found that a little chemical tweaking of the electrolyte can head off the pesky growths.
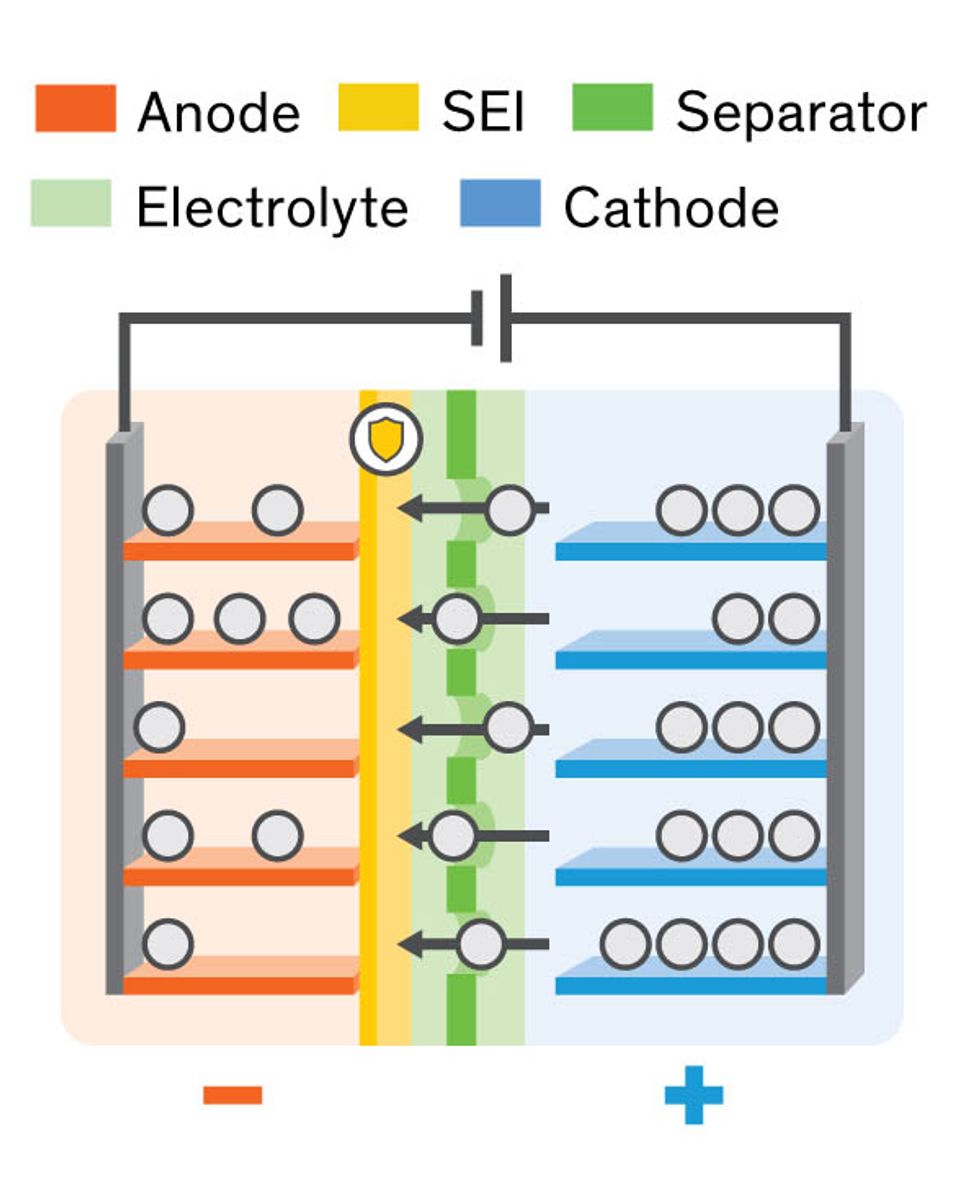
A Lithium-ion battery pack is invariably composed of one or more compartments, or cells, each of which has two electrodes covered by an extremely thin polymer film, called a separator, which prevents their coming into direct contact. Permeating the porous separator is the electrolyte, a material—today generally a liquid—that allows lithium ions to move back and forth during charging and discharging.
The slightest damage to the ultrathin separator can put the electrodes into direct contact and create an internal short circuit, which can generate enough heat to make the cell catch fire. The heat of the fire may then overheat adjacent cells, resulting in a chain reaction that can easily cause the whole battery pack to explode.
So it’s the integrity of the cell’s separator that matters most. Of course, every effort must be made during the manufacturing process to prevent damage to the separator, but even a perfectly fabricated separator can fail in operation if dendrites later damage it.
Dendrites are sharp bits of lithium metal that grow from the anode. These fibers can spread like kudzu vines into the electrolyte, pierce the separator and make their way to the cathode. It’s amazing how such tiny little things can cause so much destruction: They were responsible, for example, for the fires that grounded the worldwide fleet of Boeing 787s in 2013.
Dendrites tend to grow when the battery is overcharged, because that’s when the lithium ions migrating into the anode can no longer find a berth. Normally, the ions slip between the atomic layers of the anode, a process called intercalation, but when the space between the layers is all filled up—as can happen during overcharging—there’s nowhere else for the lithium to go but onto the surface. There they form the seeds of a metallic crystal, which grows with each new charge-discharge cycle.
Solving this problem of dendrite growth matters not just for today’s generation of lithium-ion batteries but also for future batteries that will need lithium-metal anodes. That’s because lithium metal has a high theoretical specific energy capacity—3,860 milliampere-hours per gram—and a negative electrochemical potential no other anode material can match. A higher potential allows for a higher battery voltage, which is just what’s needed in electric cars and in mobile devices.
Both these qualities make lithium anodes critical to battery technologies that are still in the lab, like the highly promising lithium-sulfur and lithium-air batteries, which can store 5 to 10 times as much energy by weight as today’s lithium-ion designs. Those future batteries may not be able to incorporate—as lithium-ion batteries do—anodes made of graphite, which has a theoretical capacity of only 372 mAh/g.
Heading Off Dendrites
The formation of lithium dendrites takes place at the meeting point between the anode and the electrolyte, in a layer called the solid electrolyte interphase (SEI). After enough lithium ions move into the anode and accept electrons there, the anode finally expands enough to break the SEI layer. From that point onward, lithium begins to form deposits at the broken part of the SEI. And these deposits seed dendrites.
Later, during discharging, lithium ions are pulled out of the anode, shrinking it again. The SEI layer collapses, generating more cracks and pinholes from which still more dendrites can begin to shoot out the next time the cell charges. Also, by exposing so much metallic lithium to the electrolyte, these cracks enable the two components to react chemically. As lithium disappears into the resulting chemical product, the lithium that remains for use in the cell diminishes. That decline lowers what’s called the coulombic efficiency, which can be determined by dividing the amount of lithium removed from use by the amount of lithium still participating in the reactions during each charge-discharge cycle.
Also, because they are very fragile, dendrites often break off from the anode, generating “dead lithium” that cannot be reused, which further lowers the coulombic efficiency of the cell. To compensate for such losses, today’s batteries must include excess lithium, which adds substantially to their weight and cost.
To head off dendrites, we need to shore up the SEI by forming a “super” SEI layer that’s uniform and stable. One way to achieve this is to modify the anode’s surface by laying down an artificial SEI layer, as it were. We’ve tried it, and it works. Unfortunately, this approach greatly complicates the fabrication of lithium-ion cells.
Another tactic is to reformulate the electrolyte by including substances that reinforce the SEI layer. The challenge is that such additives must easily dissolve, and most candidate materials don’t—a long-standing problem for battery researchers. The additives that do dissolve quickly are consumed during cycling, and as a result the SEI layers fall apart over the long term.
How, then, do you find the right additive? We got our key idea following a roundabout path.
We’d been considering the problem of the lithium-sulfur battery, the futuristic technology we mentioned earlier. What makes this combination so attractive, in theory, is its ability to store—in the same amount of mass—more than five times the energy of today’s lithium-ion batteries. Such a battery uses metallic lithium for the anode and sulfur for the cathode, and during the reactions that take place while charging or discharging there are a number of steps that create intermediate products at the sulfur cathode. These products, known as polysulfide ions, are highly soluble in the electrolyte, and that means that when the battery is in operation they can travel from the cathode, pass through the separator, and then arrive at the anode. That’s not good: Only the lithium ions ought to get that far. When these polysulfide ions hit the anode, they react vigorously with the lithium, accept electrons, and are reduced to a solid. Not only does this process slowly deplete sulfur from the system, it also gradually forms a coating that can wreck the lithium anode. This has been the main difficulty dogging the development of lithium-sulfur batteries.
To avoid this parasitic reaction, researchers have mainly tried to restrict the polysulfide from leaching out of the sulfur cathode in the first place. In one of our brainstorming sessions, we began to think different, as Steve Jobs might say: What if we could actually take advantage of this reaction? By controlling how the polysulfide ions react with lithium, perhaps we could not just form a strong and stable SEI layer but actually nip dendrites in the bud!
Meanwhile, we discovered that lithium nitrate—a very commonly used lithium salt—had long been considered as a potential electrolyte additive because of its ability to restrict—or passivate—the reactivity of the lithium metal. Perhaps by adding both polysulfide and lithium nitrate to the electrolyte we could create complementary actions: Polysulfide reacts with lithium metal, while lithium nitrate can help to prevent the lithium from reacting with polysulfide. By manipulating these two competing reactions, we should be able to turn the sulfur-lithium reaction from a flaw to a feature.
We added lithium polysulfide and lithium nitrate to the electrolyte in various concentrations. We studied the effect on the process of lithium plating and stripping in a two-electrode test cell that used lithium metal as one electrode and a stainless-steel foil as the other.
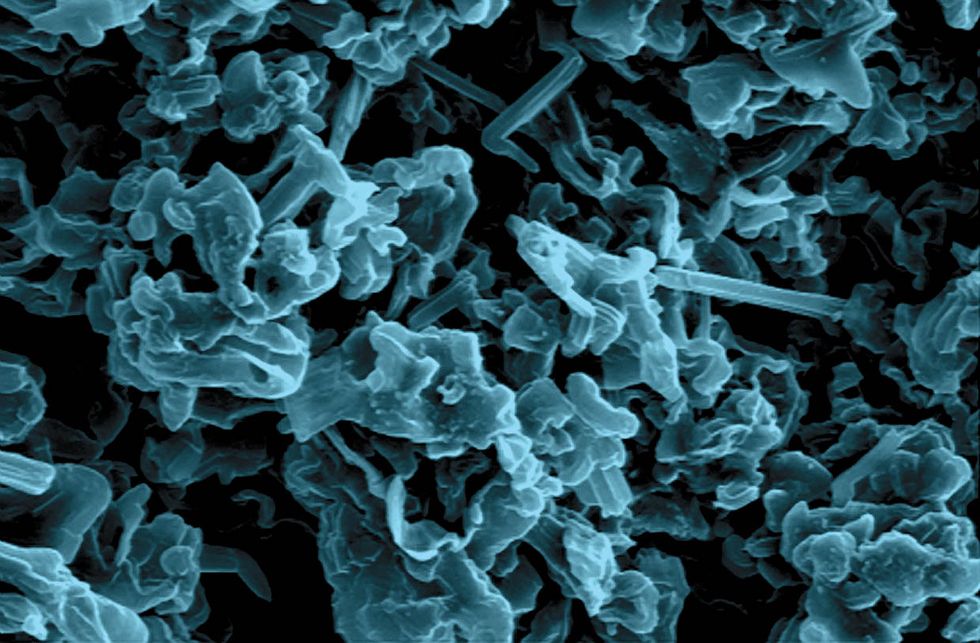
We assembled coin cells, also called button cells, similar to the ones that power small electronic devices, such as watches, calculators, and hearing aids, and we applied a constant current during charging, allowing the same current to flow during discharge. We deposited a bit of lithium onto the stainless steel by charging the cell; then we stripped it off in discharge, repeating the cycle many times. Finally, we took the cell apart and examined the lithium deposit under a scanning electron microscope.
What we saw was intriguing. Without electrolyte additives, the plated lithium formed structures that were thin, sharp, and fiberlike—dendritic, in a word. But when we added lithium nitrate to the electrolyte, the deposited lithium was thicker, less sharp, and shaped more or less like a noodle. The lithium nitrate had moderated dendrite growth but not prevented it.
Next, we added both lithium polysulfide and lithium nitrate to the electrolyte in various quantities. At just the right balance of additives, the synergistic effect we’d sought came through: No harmful dendritic structures grew. Instead we got flat, pancake-shaped lithium deposits. Even after hundreds of charge-discharge cycles, the surface of the plated lithium was still flat, without any dendritic structures.
Besides heading off dendrites, our two additives together greatly enhanced the coulombic efficiency and the cycling stability. The coulombic efficiency was better than 99 percent over more than 300 charge-discharge cycles. Charging caused plating on only a tiny bit more lithium than was stripped off during discharge. In contrast, with lithium nitrate alone, coulombic efficiency drops to less than 92 percent after just 180 cycles, and with polysulfide alone it’s only about 80 percent.
These two additives, working together, bring a huge improvement because of their effect on the SEI layer. To figure out the exact mechanism of that effect, we used a technique called X-ray photoelectron spectroscopy and also conventional electron microscopy to deduce the structure and chemical composition of the SEI layer. In cells using one or the other additive, we found that the SEI layers were marred by lots of cracks and pinholes. When both additives were present, though, we got a flat, uniform SEI. And the chemical breakdown of the SEI layers confirmed that the two additives indeed had competing effects.
When we added both lithium nitrate and polysulfide, the lithium nitrate was the first to react with the lithium metal, and it did passivate the metal’s surface, as expected, drastically reducing the metal’s reaction with the polysulfide. The product from the first reaction formed mainly in the upper layer of the SEI, and it effectively suppressed the formation of dendrites.
This technique for preventing the growth of dendrites is still in its early days. We have problems to solve before we can think about commercialization. A particular difficulty is finding the precise formulation of the electrolyte additives for each of the several different kinds of lithium batteries.
But this new strategy holds out the promise not only of creating a safer, higher-energy lithium-ion battery but also of paving the way for next-generation battery chemistries.
With dendrites defeated, a lithium-metal design could store far more energy than today’s batteries while lasting through the many charging cycles that consumer products require. We predict that in another 5 to 10 years, our technology will allow the commercialization of safe, superhigh-capacity batteries for phones, laptops, cars, and airplanes. That would make headlines for rechargeable batteries of a much more positive sort.
About the Authors
Weiyang Li is an assistant professor of engineering at Dartmouth University. Yi Cui is a professor of materials science and engineering at Stanford University.