Bioengineers Aim to Break Big Ag’s Addiction to Fertilizers
Designer microbes could replace the chemical fertilizers that contribute to climate change


Jump to Articles in this Section
Big Ag is addicted to nitrogen fertilizers. It’s a massive problem for the global climate, yet it may yield to a microscopic solution: microbes rewired to “fix” nitrogen from the air and turn it into a natural type of fertilizer that corn, wheat, and other cereal crops can use.
Until the Green Revolution changed agriculture in the mid-20th century, farmers fed cereal crops either by spreading nitrogen-rich manure on their fields or by planting a legume crop (such as beans or peas) whose root systems contain microbes that naturally nab atmospheric nitrogen, and then plowing that crop under to fertilize the cereal crop they actually wanted to grow. But these inefficient methods couldn’t begin to support the 7 billion people alive today. The Green Revolution ushered in a new era of chemical fertilizers, enabling farmers to feed the booming global population—but also creating a dangerous addiction.
And so, every year, the world’s farmers lavish on their crops some 120 million metric tons of nitrogen fertilizer made via the century-old Haber-Bosch process. This industrial operation requires high pressure and temperature, so fertilizer factories burn a lot of fossil fuel, releasing carbon dioxide right away; later, the unused fertilizer in the soil returns to the air as nitrous oxide (N2O), a gas that has 300 times as much heat-trapping power as carbon dioxide. The combined emissions from fertilizer production and use are equivalent in their effect to as much as 1.3 billion metric tons of CO2 a year.
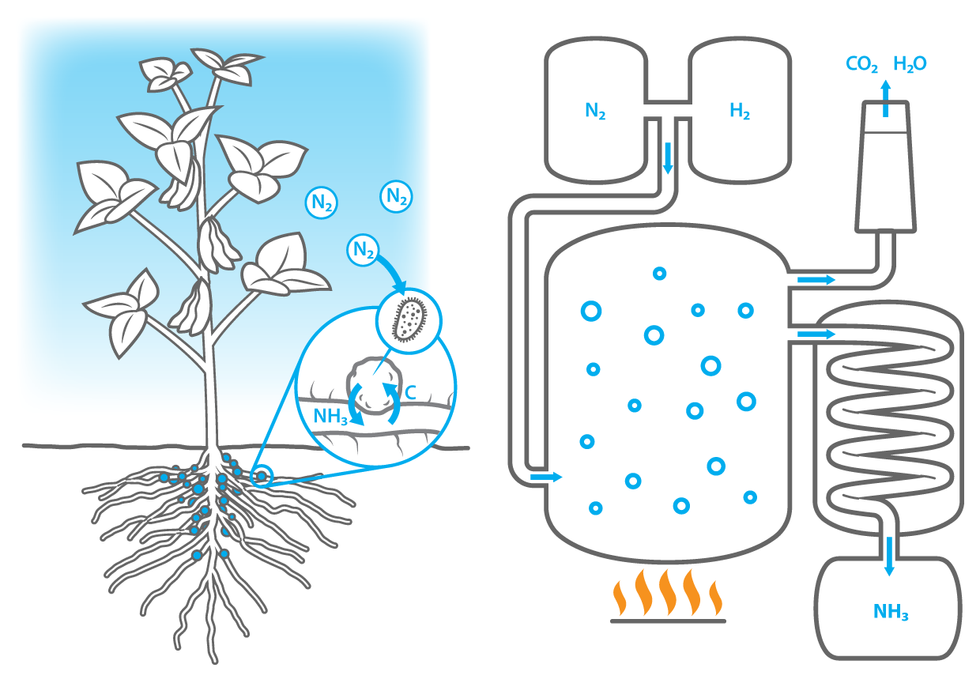
How to Feed a Plant
Nature’s Way: In the root nodules of a bean plant [left], nitrogen-fixing bacteria use enzymes to convert atmospheric nitrogen into ammonia. The bacteria give that essential nutrient to the plant in exchange for carbon-based sugars.
Industry’s Way: In a fertilizer factory, the Haber-Bosch process combines atmospheric nitrogen with hydrogen from natural gas to produce ammonia. The reaction chamber requires temperatures of 400 °C (752 °F) or higher and pressures upwards of 20 megapascals (200 atmospheres). The factory uses a huge amount of electricity to maintain these conditions, and also emits CO2 as a by-product of the reaction.
Biologists have long sought a better, cheaper, and more environmentally friendly way to fix nitrogen. They’ve tried to make cereal crops form symbiotic relationships with nitrogen-fixing bacteria, as legumes do. They’ve tried to convince bacteria such as Azospirillumand Klebsiella to set up shop in the roots of wheat and rice plants. Yet, despite more than half a century of effort, no one has yet managed to endow any of the world’s major grains with a viable nitrogen-fixing bacterial partner.
Enter Joyn Bio, a Boston-based spinoff launched last September from two companies: Bayer CropScience, which boasts a vast library of agricultural microbes, and Ginkgo Bioworks, a pioneering biotech firm that creates custom-made bacteria for industrial applications.
Ginkgo’s Boston research hub is home to an assembly line of robots that are programmed to manufacture, read, or edit strands of DNA. Joyn’s idea is to synthesize variations on some of the genes that are believed to play a role in nitrogen fixation, including those involved in the cooperation between legumes and the bacteria specific to their root systems. Snippets of this synthetic DNA are then slotted by machines into microbes growing in rows of tiny fermentation chambers, before another set of automated tools characterizes the genetically altered microbes’ performance every which way. Synthesize, build, test, repeat.
Joyn is hoping this engineering approach to the fertilizer-replacement challenge—combined with computational power to integrate terabytes of data into predictive metabolic models—will make the company succeed where others have failed. “Our goal is to use all the tools of synthetic biology to take naturally occurring microbes that have evolved in plants, and see what we can do in the lab to create organisms that replace significant amounts of fertilizer for crop plants,” says Johan Kers, head of nitrogen-fixation research at the company. “If we can do that, we’ll have a big party.”

They may have a lot of guests to invite. Joyn is structured like a scrappy startup, with fewer than 20 full-time employees split between research sites in Boston and West Sacramento, Calif. But as a joint venture between Bayer—which will become the world’s largest supplier of seeds and crop chemicals after its US $62.5 billion buyout of Monsanto—and Ginkgo, one of only a handful of private biotech companies valued at more than $1 billion, the spinoff has ample resources.
The lab space and microbial production capacity comes from Ginkgo, the bacterial strains and greenhouses from Bayer. The parent companies, together with a hedge fund, also put in $100 million to bankroll Joyn’s R&D operations over the next five years. “These things all came in on day one, so we’re really hitting the ground running,” says Joyn CEO Mike Miille.
Joyn has already sequenced the genomes of around 20 different bacterial species, all of which naturally take gaseous nitrogen from the air and use enzymes to convert it into ammonia, which plants use to make DNA, proteins, and other essential building blocks of life. Some of these critters use the same well-characterized enzymes found in the nitrogen-fixing bacteria that live in the root nodules of legume plants, differing only in that they don’t naturally share their biochemical bounty with crops. Others may use weird new enzymes that have yet to be discovered because no one has ever embarked on this kind of screen. “We’re sampling the solution space for this engineering problem,” Kers says.
Once Kers and his team close in on a few enzyme-encoding genes of interest, the next steps will largely be outsourced to Ginkgo’s foundries, so named to evoke metallurgic factories that manufacture metal parts to exacting specifications. At Ginkgo HQ, these foundries form a glass-encased core that expanded late last year and now spans the length of two football fields. There, software and robotics automate much of the drudge work of organism design.
That kind of process engineering and rapid prototyping is unparalleled in the biotech industry, says Paul Miller, chief scientific officer of Synlogic, a Boston-area company that’s partnering with Ginkgo to develop microbial therapeutics for diseases. “Ginkgo is really a world leader when it comes to a massively parallel engineering capability and the ability to iterate around organism-design ideas on a large scale,” Miller says.
For Joyn, the tight relationship with Ginkgo means it can build hundreds of engineered microbes with slight variations in one or more genes. Foundry scientists, called organism engineers, can test the performance of each microbe through chemical analyses on a mass spectrometer. Kers and his colleagues can study the data, input it into their model, and order a new batch of engineered bugs. Those that look promising are shipped off to West Sacramento for further evaluation alongside corn plants raised in greenhouses and, eventually, in the fields.

Boosters of synthetic biology think the design-build-test framework that has proven itself in Silicon Valley will also work in microbial engineering. “These are some of the smartest and most talented people in the business,” says Andrew Hessel, a biotechnologist who until recently worked as a researcher at Autodesk Life Sciences, which builds software for biological design. “People have been trying to hack this forever, and now they actually have the tools to do it for real.”
But nitrogen fixation is not merely an engineering challenge; it’s also an ecological one. “And boy, has it proven tricky,” warns Allen Good, a plant scientist at the University of Alberta, in Canada. “It’s one thing to take a piece of DNA and put it in bacteria and get it to fix nitrogen,” he says. “But to build a symbiotic relationship is so much more complex than that.”
In the root system of a bean plant, bacteria supply the plant with ammonia and receive sugar in return. Both sides profit—that’s the essence of symbiosis. But if you engineer a strain of bacteria to give ammonia away, you force it to incur a cost that nonengineered bacteria don’t shoulder. Naturally occurring microbes may therefore outcompete the engineered ones, eliminating them quickly. You could get around the problem by engineering symbiosis—by getting the plant to reciprocate—but that isn’t easy to do. Cereals and nitrogen fixers don’t play nice together. And, like a teacher trying to cajole a classroom full of selfish toddlers to share their toys, scientists have struggled to promote cooperation in the soil. “You really need to have a signal exchange between partners—between the plant and the microbe,” says Philip Poole, a plant microbiologist at the University of Oxford.
In addition to the scientific challenge of engineering microbes and plants, there’s also a societal one: Consumers are still distrustful of genetically modified organisms (GMOs) in foods, and the designation brings additional regulatory scrutiny. There are, however, ways of using the tools of synthetic biology that tiptoe up to the GMO line without crossing it—for example, by mutating bugs at random and then selecting for the best ones. That’s the strategy of Pivot Bio, one of the few other companies developing nitrogen-fixing bugs. Pivot, based in Berkeley, Calif., starts with microbes that can naturally capture airborne nitrogen but fail to do so in agricultural settings. The company characterizes these critters with all the fanciest genomic tools available, building computational models to better understand gene circuitry, then tries to breed progeny that don’t have the feedback mechanisms that normally shut off nitrogen fixation in fertilizer-rich soils.
“My team has the best synthetic biologists in the world, and they can do the craziest transgenic things out there,” says Pivot’s CEO and founder, Karsten Temme. “But we’ve put on handcuffs and said we’re not going to build transgenic microbes because that’s not culturally acceptable, and it means you have to go through a regulatory process to get approvals.”

Not so Joyn. According to Brynne Stanton, head of metabolic engineering at the company, Joyn will use all the synthetic biology tools at its disposal. Only later, if Joyn succeeds in engineering a robust nitrogen-exchanging symbiosis with corn, will the company see if it’s possible to get to the same end products in a way that doesn’t get them slapped with a GMO label. “We are really starting with a blank slate,” says Stanton, as she sips from a can of coconut water that bears the words “non-GMO” on its label.

Smil Says…
Unfortunately, symbiosis does not come free; legumes pay a considerable price for sharing their photosynthetic products with bacteria.
Click here for Vaclav Smil”s commentary on the entire Blueprints for a Miracle special report.
Many leading experts, even some who work with Pivot, applaud this approach. “To be able to reach a product that’s not GMO—at this point, I don’t see how that would be possible,” says Jean-Michel Ané, who studies plant-microbe interactions at the University of Wisconsin–Madison and serves on Pivot’s scientific advisory board. In his academic research, Ané is coleading a $5.1 million project called Synthetic Symbioses, which is taking the genetic engineering strategy one step further: modifying DNA of both corn and a nitrogen-fixing microbe so they’re fully reliant on each other. Others, like Luis Rubio, a biochemist at the Technical University of Madrid, are trying to cut the microbe out of the equation entirely and simply engineer the ability to fix atmospheric nitrogen into the plants themselves, which would then be self-fertilizing.
Whatever works, the initial commercial products from Joyn or its rivals will likely displace only small amounts of chemical fertilizer, maybe 10 to 20 percent, executives say. That modest reduction might help limit local impacts, such as air and water pollution, but “it wouldn’t make much of a dent in global N2O emissions unless combined with other nitrogen best-management practices,” says David Kanter, an environmental scientist at New York University who studies nitrogen pollution.
These companies have to start somewhere, though—and in Pivot’s case, that means deploying its nitrogen-producing microbes alongside traditional fertilizers in large-scale field testing taking place this growing season at farms across the U.S. corn belt. “Eventually,” says Pivot’s Temme, “we want to replace all the fertilizer.”
Miille, of Joyn, has equally lofty ambitions. “Nobody is sitting here saying this is easy. There are a whole bunch of things that are unpredictable,” he says. But, he adds, “this is really going to push the technical boundaries forward.” As his company’s organism engineers work through their design-build-test cycle, they just might find an unpredictable little microbe with big potential.
This article appears in the June 2018 print issue as “Breaking Big Ag’s Fertilizer Addiction.”