We have seen batteries get so small that their anodes consist of a single nanowire. In that instance, the research was not really aimed at creating a new battery design but instead at demonstrating that the researchers could use a liquid electrolyte in the vacuum of a transmission electron microscope (TEM).
In contrast, researchers at the University of Maryland have their focus on building a new type of battery that is based on tiny nanopores in a ceramic material. The researchers have developed a method for introducing an electrolyte into the nanopores so that each cavity acts as an individual battery cell and all of them are joined in parallel.
Commenting on their research, published in the journal Nature Nanotechnology, the Maryland researchers note that: “a single nanopore structure that embeds all components of an electrochemical storage device could bring about the ultimate miniaturization in energy storage.”
In the video below, one of the authors of the paper, Chanyuan Liu, explains that the battery they developed in the lab can undergo one thousands charge/discharge cycles. It can be fully charged in 12 minutes, she adds in the press release.
Eleanor Gillette, another member of the group, says in the video that the aim is to develop the manufacturing technology to make larger nanopore structures possible. Liu says that they have already identified ways to increase the power of the batteries by ten times.
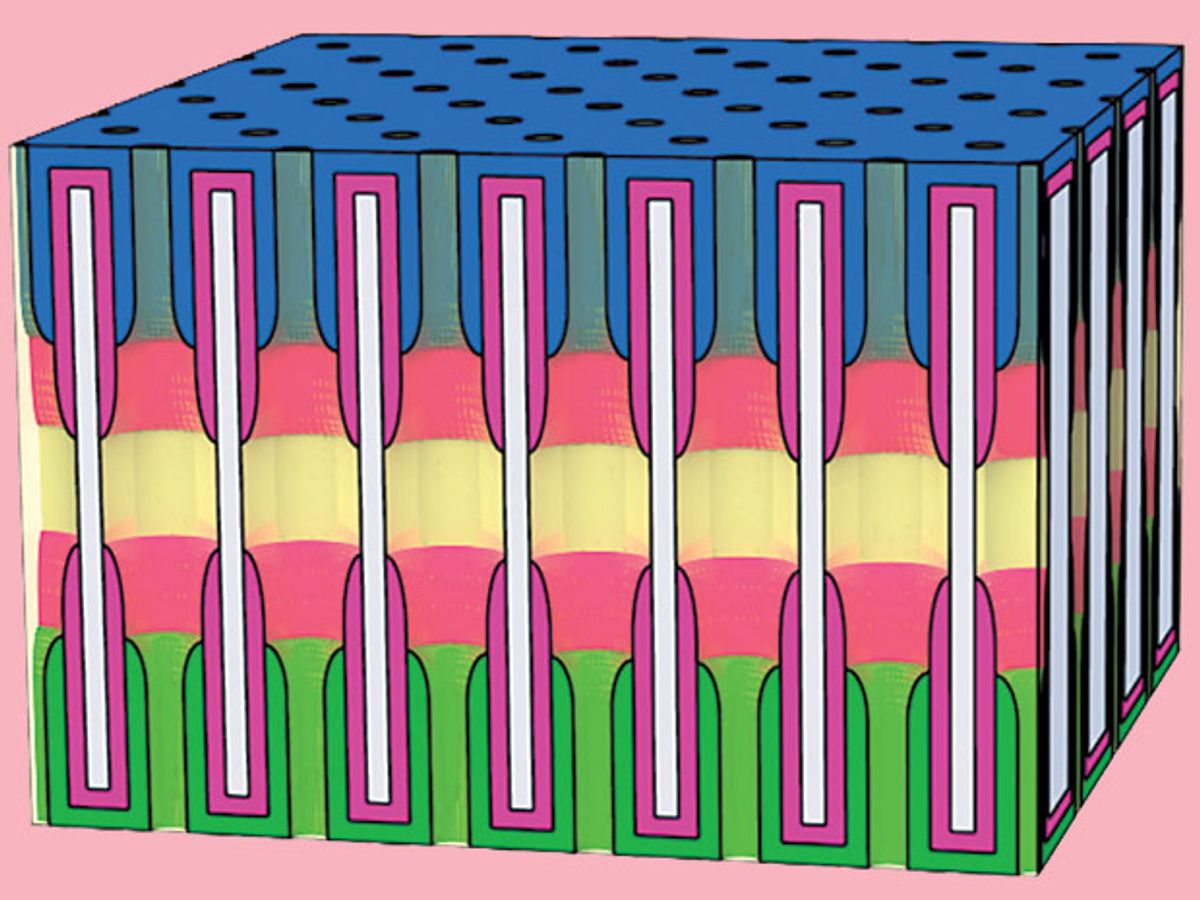
The entire design of the battery involves each of its nanobattery components being composed of an anode, a cathode, and a liquid electrolyte confined within the nanopores of anodic aluminium oxide, which is an advanced ceramic material. Each nanoelectrode includes an outer ruthenium nanotube current collector and an inner nanotube of vanadium pentoxide storage material. These together form a symmetric full nanopore storage cell with anode and cathode separated by an electrolyte region. The vanadium pentoxide is treated with lithium at one end to serve as the anode, with pristine vanadium pentoxide at the other end serving as the cathode.
The researchers believe that the key to the success of the design is the uniformity in shape and size of the nanopores, which allows for a dense packing of the nanopores into the ceramic material.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.