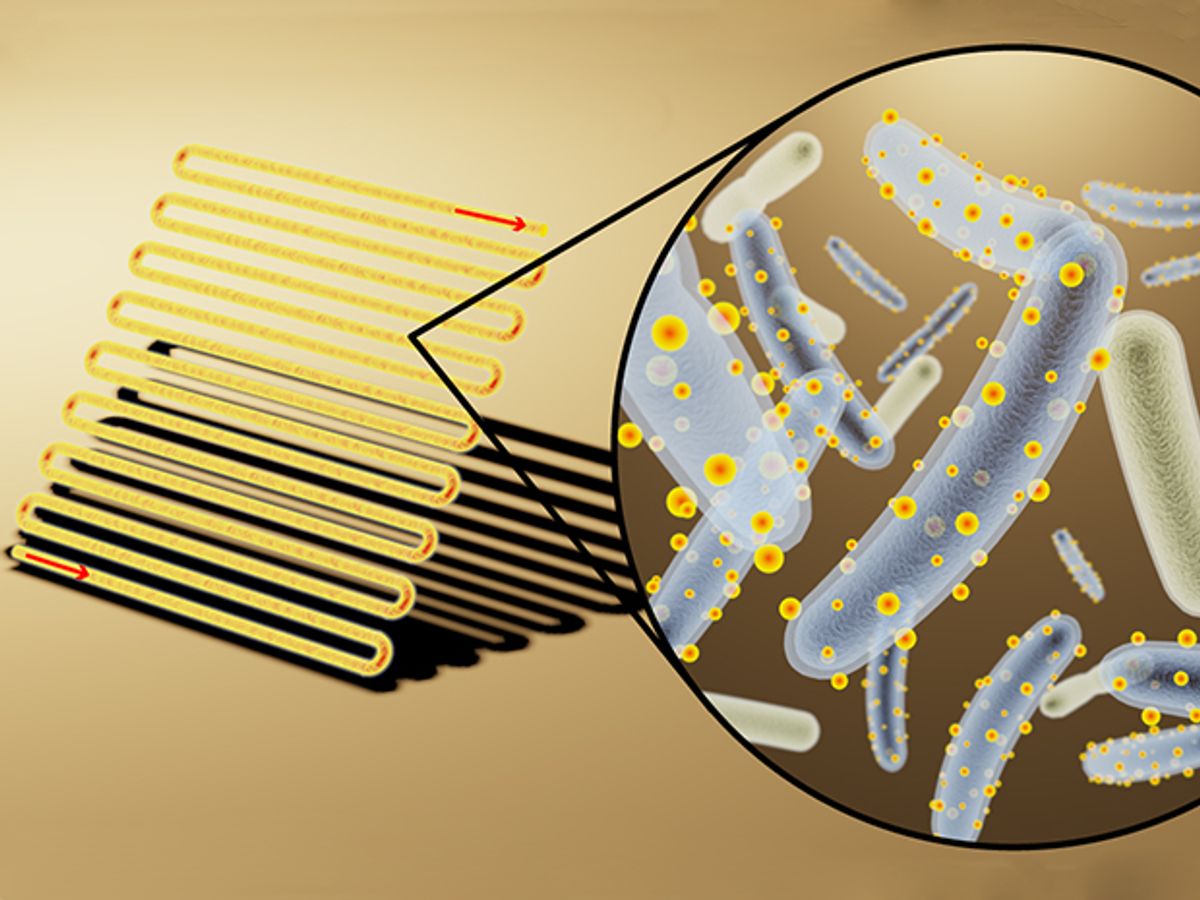
Researchers at the University of California Berkeley may have introduced a new era in artificial photosynthesis. They’ve devised a way to cover bacteria with cadmium sulfide semiconductor nanocrystals to breakdown carbon dioxide (CO2) into acetic acid, a precursor to a host of other products.
In research that is being presented at the 254th National Meeting & Exposition of the American Chemical Society (ACS), Kelsey K. Sakimoto, a researcher at the lab of Peidong Yang at Berkeley, has demonstrated that the non-photosynthetic bacterium known as moorella thermoacetica can self-assemble into nanocrystal-clad cyborgs that essentially supercharge the bacteria into CO2-reduction powerhouses.
“It's actually a natural, overlooked feature of their biology,” explains Sakimoto in an e-mail interview with IEEE Spectrum. “This bacterium has a detoxification pathway, meaning if it encounters a toxic metal, like cadmium, it will try to precipitate it out, thereby detoxifying it. So when we introduce cadmium ions into the growth medium in which M. thermoacetica is hanging out, it will convert the amino acid cysteine into sulfide, which precipitates out cadmium as cadmium sulfide. The crystals then assemble and stick onto the bacterium through normal electrostatic interactions.”
While Sakimoto and his colleagues were able to leverage an overlooked property of these bacteria, they remained somewhat uncertain of how the bacteria operated in its superpower cyborg state.
A quick primer on photoelectrochemical reduction of CO2: Semiconductor materials have a bandgap that generates electron-hole pairs when they are struck by photons with energy levels that are higher than the bandgap of the semiconductor. This is how semiconductor materials convert solar energy into electrical energy in photovoltaic devices, and it forms the basis of this approach to CO2 reduction.
The northern branch of the Department of Energy’s Joint Center for Artificial Photosynthesis (JCAP), located at Berkeley National Laboratory, has been focused on this line of research since 2015. (IEEE Spectrum visited these labs in May of this year to get a full rundown on the status of the research.)
In this latest research, Sakimoto and his colleagues conducted spectroscopic investigations which revealed that enzymes in the bacteria's membrane associate and attach themselves to the nanocrystal. When the cadmium sulfide is excited with light, the excited electron from the conduction band is injected into the enzyme, which produces some sort of biological electron carrier (like H2) that is used for the CO2 fixation pathway. “The position of the conduction band is crucial as it must be high enough to pass into the enzyme; we think it's hydrogenase,” adds Sakimoto.
You can see a visualization of this process in the video below.
The nanocrystal-clad bacteria are able to achieve a high breakdown efficiency of up to 84 percent, overcoming the issue of a CO2 reduction in which a soup of byproducts results from the process. While this approach doesn’t lead to a synthetic fuel, like ethanol, it does produce just one product: acetic acid.
“While acetic acid isn't terribly useful on its own, other bacteria love it,” says Sakimoto. “We have strains of engineered E. coli that are engineered to eat the acetic acid and produce butanol, the bioplastic polyhydroxybutyrate, and a family of pharmaceutical compounds. We think acetic acid is an ideal intermediate product as most engineered strains of bacteria can use it to really make any product under the sun.”
As Frances A. Houle, deputy director for science and research integration at JCAP North told us during our lab visit last May, in the field of artificial photosynthesis, the real challenge still is using it for CO2 reduction. While a lot of progress is being made on the chemical catalysis front, as evidenced by research that came out of Berkeley’s Material Science Division last month, Sakimoto believes that nothing at this point beats biology. “For now, we are pioneering this semi-artificial photosynthesis process as the only pathway towards complex carbon products,” he adds.
In order to push this towards commercially viable systems, Sakimoto and his colleagues are interested in identifying other amenable semiconductors. To that end, the researchers are looking for semiconductor nanocrystals beyond cadmium sulfide, which currently serves as a nice starting point because it's well studied and relatively easy to make.
Sakimoto adds: “Ultimately, we need to push these bacteria to make higher performing semiconductors, like silicon, or III-V semiconductors like indium phosphide, small band gap sulfides like maybe lead sulfide, or even more exotic ones like ternary semiconductors such as CZTS. We are also keeping an eye on the biological end, and trying to understand how to better design and engineer the association of these membrane enzymes to the semiconductor surface.”
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.