Porous crystals could scrub carbon dioxide from industrial fumes for half the energy often needed now, scientists say. These novel materials could also help clean the air in submarines and on the International Space Station.
All in all, these new materials could slash the amount of energy now consumed in carbon capture by half or more, researchers at the University of California, Berkeley say. They detailed their findings in the March 12 issue of the journal Nature.
Power plants often burn coal and other fossil fuels, releasing the greenhouse gas carbon dioxide. Power plants designed to stem their carbon dioxide emissions typically capture the gas by bubbling their fumes through water loaded with alkaline amines. To release the gas for sequestering underground, the liquid is heated up to 150 degrees Celsius. The entire process is expensive, consuming about 30 percent of the power generated.
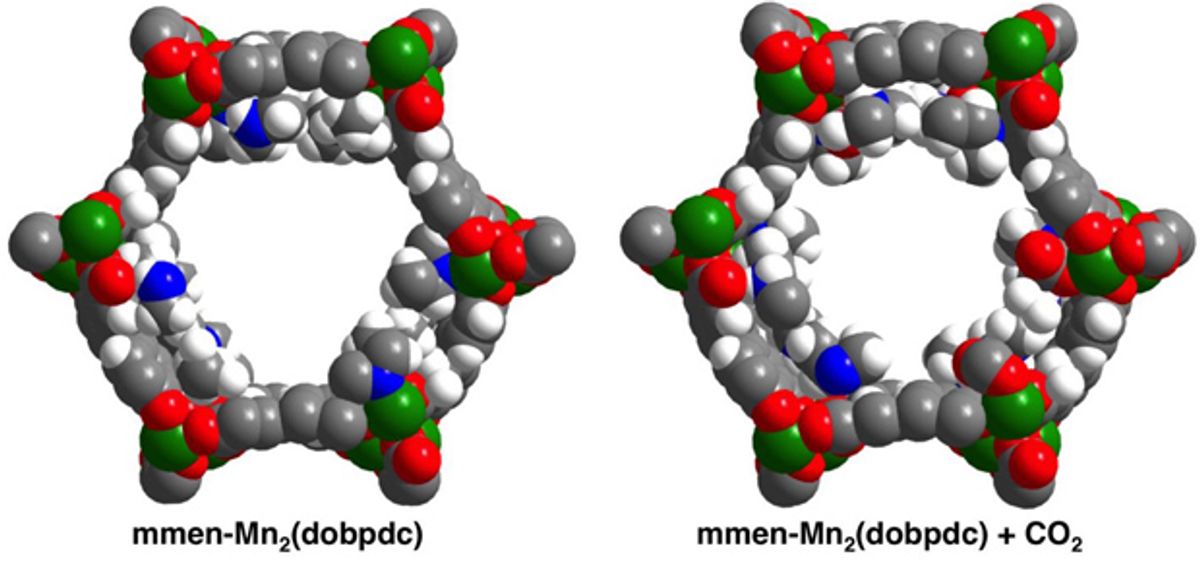
Instead of using amine solutions, scientists Berkeley and their colleagues investigated crystals known as metal-organic frameworks (MOFs). These materials combine metal nodes with organic linker molecules, and are riddled with microscopic channels.
The researchers took MOFs and modified them with nitrogenous compounds known as diamines. They found that carbon dioxide can quickly both load onto and come off these porous crystals at specific temperatures and pressures.
The scientists can tune these crystals to capture carbon dioxide at various temperatures, such as the 40-degrees Celsius heat of fumes from a power plant or the room-temperature air of a submarine or a space station. These materials can then release the carbon dioxide when heated by only about 50 degrees Celsius instead of the 80 to 110 degrees Celsius required in conventional carbon capture. In addition, because these compounds are solid, they also do not require the huge energy costs required to heat the water used in standard carbon scrubbing techniques.
Charles Q. Choi is a science reporter who contributes regularly to IEEE Spectrum. He has written for Scientific American, The New York Times, Wired, and Science, among others.