Since 2012, IEEE Spectrum has been covering Domenico Pacifici at Brown University as he works to improve the capabilities of plasmonic interferometers. One major application would be glucose monitors that enable diabetics to check glucose levels through saliva instead of blood—no finger pricking necessary.
In his latest research, Pacifici and his team have developed a way to get a plasmonic interferometer to take measurements without the need for a coherent light source.
To have a coherent light, the light waves have to run in parallel, possess the same wavelength, and travel in-phase, which means the peaks and valleys of light waves are in alignment. Producing this kind of light demands expensive and bulky equipment. By eliminating that need, Pacifici’s team has created a far smaller and less expensive way to operate these devices.
“It has always been assumed that coherent light was necessary for plasmonic interferometry,” said Pacifici, in the press release. “But we were able to disprove that assumption.”
In research published in the Nature journal Scientific Reports, Pacifici and his team embedded light emitters in the form of fluorescent atoms directly into the sub-wavelength cavities of the plasmonic interferometers. The result was that even when the source light had very low coherence, the internal emitters could get the interferometer to operate as though the light was coming from a coherent light source.
Plasmonic interferometers operate on the same principles of plasmonics as every other plasmonic device does. When photons of light hit a metal surface, they rattle the electrons in the metal so much that they generate waves of electrons known as surface plasmons.
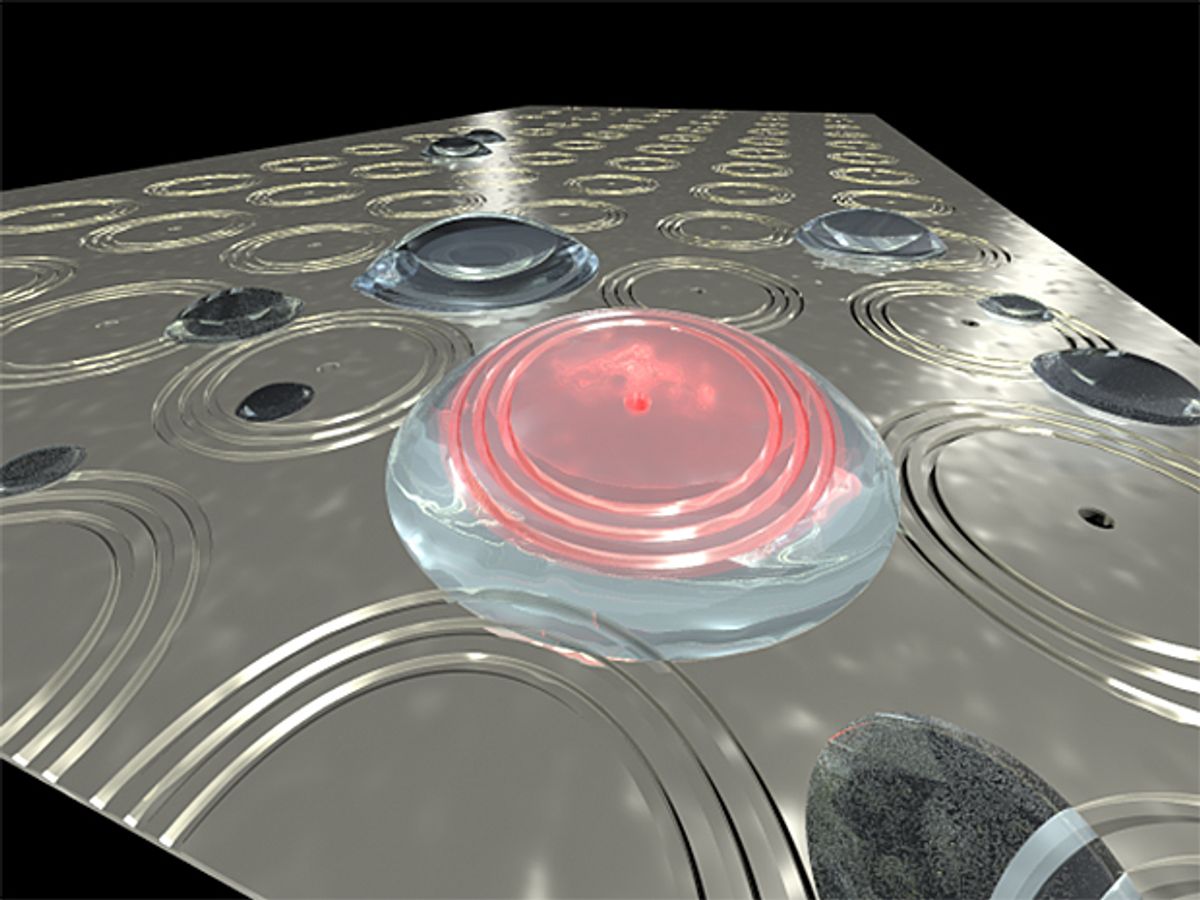
A plasmonic interferometer exploits this phenomenon by its very architecture, essentially a piece of metal that has a hole—or cavity—at its center and around that hole is carved co-centric grooves. The cavity is around 300 nanometers in diameter and the co-centric grooves are measured in microns.
When the light hits the surface of this device, some of the photons go into the cavity at the center of it while others hit the outer grooves and scatter. The scattered photons excite the electrons on the metal surface to the point where they become waves of surface plasmons. Just like waves on water, the waves move along the surface of the metal until they go into cavity at the center. Here they interfere with the photons that were originally drawn into the cavity generating an interference pattern: you can measure how the light weakens and strengthens coming out of the cavity.
By embedding fluorescent atoms in the cavity of the device, Pacifici’s team made the cavity produce its own surface plasmons. In this way, surface plasmons move out of the cavity onto the surface and then bounce off the co-centric grooves and back into the cavity. When these surface plasmons come in contact with the fluorescent atoms that were its source, it creates an interference with the directly transmitted photon. In this arrangement, the photons in the cavity and the plasmons are coming from the same emitter, so they are naturally coherent and interference occurs even though the emitters (the fluorescent atoms) are excited by incoherent light.
“The important thing here is that this is a self-interference process,” Pacifici said in the release. “It doesn’t matter that you’re using incoherent light to excite the emitters, you still get a coherent process.”
In addition to being able to use incoherent light sources, the architecture provides additional benefits, such as greater accuracy and the internal emitters mean that more delicate samples can be tested.
While this work is really just a test of concept, Pacifici believes that this is such a fundamentally different way for these devices to operate that it represents a significant breakthrough in the field.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.