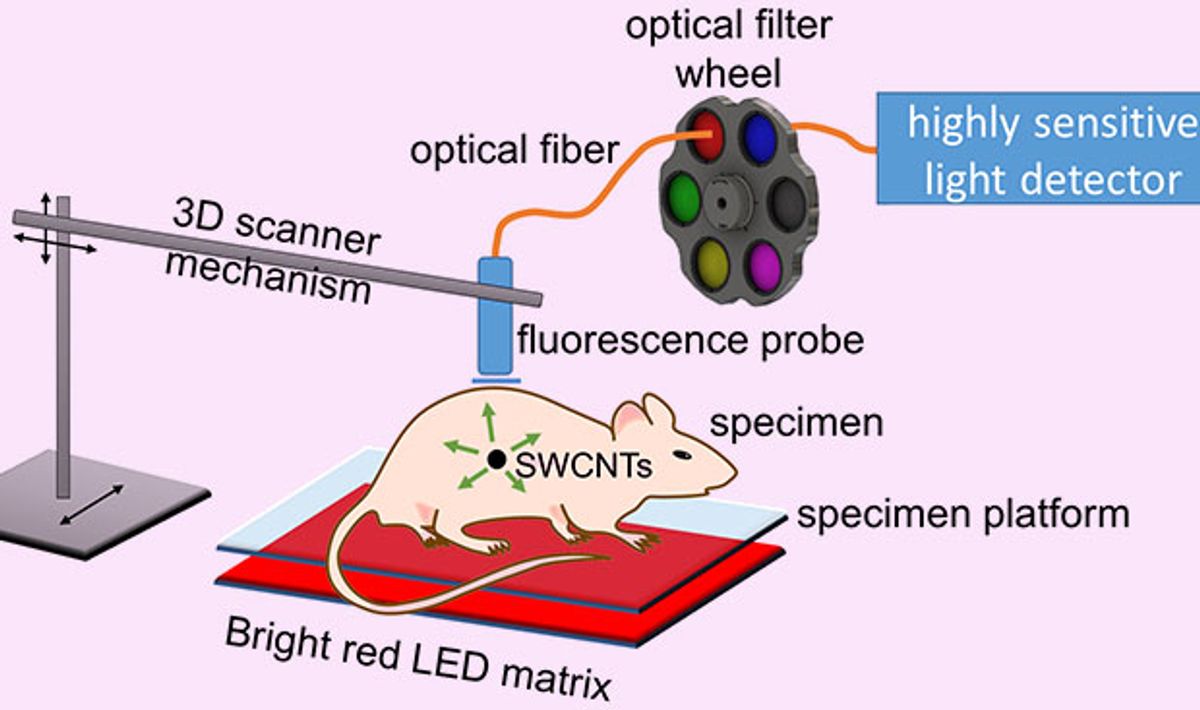
Researchers at Rice University in Houston, Texas, have developed a medical imaging technique that combines carbon nanotubes, LED light, and a photodiode detector to pinpoint the location of tumors buried 20 millimeters deep in simulated tissue. The researchers believe that this is the deepest that carbon nanotubes have been detected inside of tissue.
In research described in the journal Nanoscale, the Rice team exploited the ability of single-walled carbon nanotubes (SWNTs) to luminesce in the short-wave infrared (SWIR) region of the spectrum. While this SWNT luminescence has previously proven effective in illuminating internal organs, researchers were still unable to reliably detect and localize the source of the SWIR emission from inside tissues. The answer they came up with is a method called spectral triangulation.
This technique involves exciting the SWNTs embedded in the tissue by shining LED lights on the tissue. The light causes the nanotubes to luminesce; the infrared emissions are picked up by a scanning fiber optic probe that is connected to an indium gallium arsenide avalanche photodiode detector.
“We’re using an unusually sensitive detector that hasn’t been applied to this sort of work before,” said Bruce Weisman, the Rice chemistry, materials science, and nanoengineering professor who led the research, in a press release. Says Weisman:
This avalanche photodiode can count photons in the short-wave infrared, which is a challenging spectral range for light sensors. The main goal is to see how well we can detect and localize emission from very small concentrations of nanotubes inside biological tissues. This has potential applications in medical diagnosis.
The method the Rice team came up with departs from previous approaches in some innovative ways. First is the use of LEDs to excite the SWNTs. Typically, lasers are used to do this, but the laser beams can’t be focused inside of the tissues because of scattering. “We bathe the surface of the specimen in unfocused LED light, which diffuses through the tissues and excites nanotubes inside,” Weismann explains.
Another interesting variation from earlier work is the technique used to determine just how deeply embedded the SWNTs are inside the tissue. The fiber optic probe touches the surface of the tissue and takes readings along grid points that make it possible to determine the X and Y coordinates of the nanotubes. But to home in on the depth, or the Z coordinate, they take advantage of the way water in the tissue absorbs some of the light emitted by the nanotubes.
Weisman explained:
We make use of the fact that different wavelengths of nanotube emission are absorbed differently going through tissue. Water (in the surrounding tissue) absorbs the longer wavelengths coming from nanotubes much more strongly than it does the shorter wavelengths.
This means that, for nanotubes close to the surface, the long and the short wavelength emissions are similar in intensity because there is less tissue between the nanotubes and the detector to absorb the longer wavelengths. Accordingly, the farther the nanotubes are from the surface, the lower the intensity of long wavelengths reaching the detector. “So the balance between the intensities of the short and long wavelengths is a yardstick to measure how deep the source is,” says Weisman. “That’s how we get the Z coordinate.”
Researchers at the University of Texas MD Anderson Cancer Center, also in Houston, are testing the detector.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.