In 2011, Gert-Jan Oskam lost the ability to walk. Twelve years later, neuroscientists at École Polytechnique Fédérale de Lausanne (EPFL), in Switzerland, have helped him get back to his feet.
Oskam, who suffered a traumatic cervical spine injury from a cycling accident, regained control of his legs with the help of scientists at EPFL’s NeuroRestore research center led by Grégoire Courtine, a professor of life-sciences engineering. Courtine and his collaborators, who research and develop neurotechnologies for physical assistance and rehabilitation, implanted Oskam with a device that partially replaces the functions lost to his damaged spine.
In a recent paper published on 24 May in Nature Neuroscience, Courtine and his group present the details of Oskam’s recovery. Injuries like those Oskam experienced are the result of damage to the tissue in a person’s spine, cutting off neural communication from the brain to the rest of the body. Without that link between the nervous and the muscular systems, a person would not be able to move as they may intend to. In Oskam’s case, the injury to his cervical spine effectively disconnected his brain from his legs, which prevented him from walking.
“Digital Bridge” Allows Paralyzed Man to Walkyoutu.be
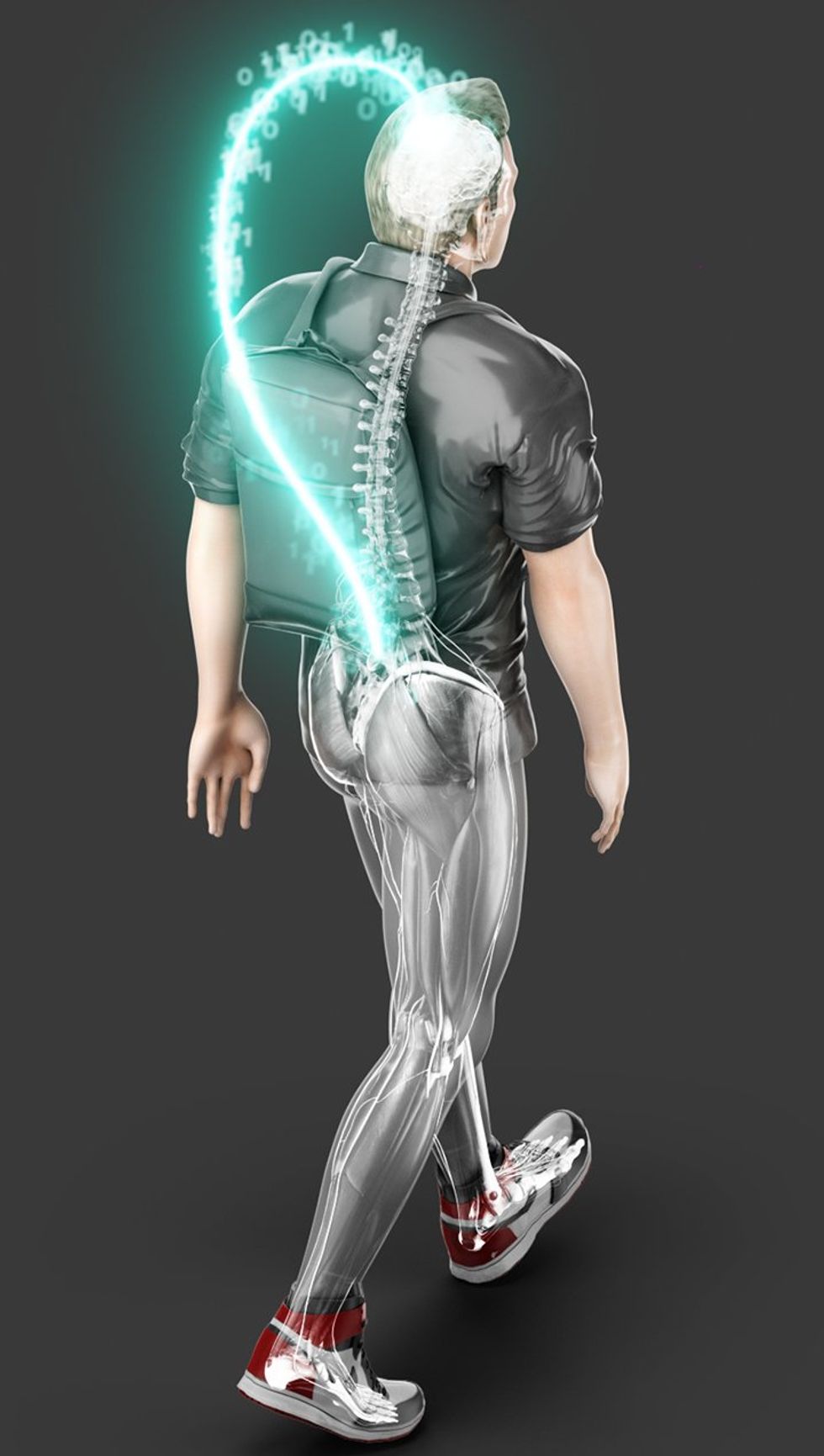
Courtine’s research presents a solution to this problem in the form of a brain-spine interface, or BSI. The BSI works in two steps. First, a brain-computer interface measures neural activity from electrodes placed on the surface of Oskam’s brain. Brain activity associated with leg control, decoded by a machine-learning model trained to recognize it, is then used to control a separate set of electrodes implanted into Oskam’s spine. This spinal stimulator activates groups of neurons driving naturalistic walking movements. In other words, the BSI bridges the communication gap in Oskam’s nervous system that resulted from his injury, giving him back his ability to walk. With the new system, he can now walk over 200 meters in a day and can stand for 3 minutes without using a walker for support.
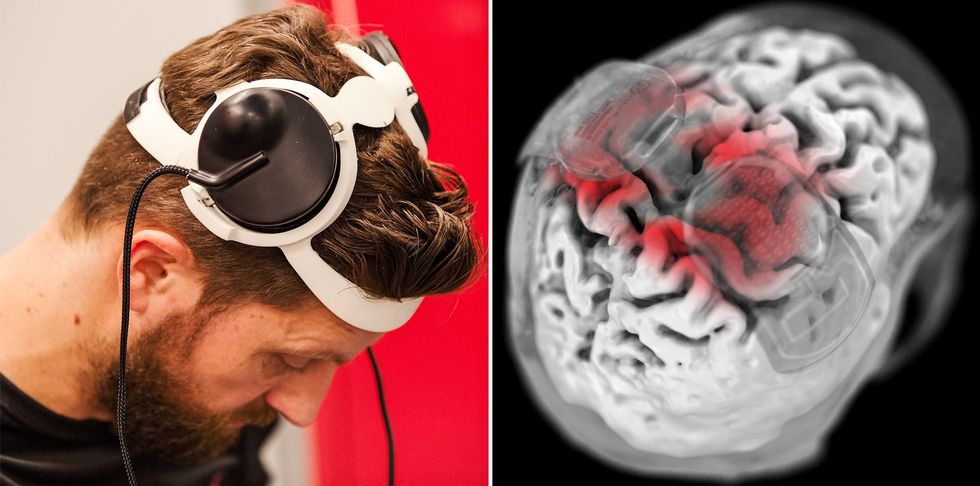
Oskam was a participant in this same group’s previous research developing the spinal-stimulation technology used in the current BSI. Previous devices were able to recreate naturalistic walking movements in Oskam’s legs, coordinating the contraction of the many muscle groups in his hips, thighs, calves, and feet. While this motion did feel somewhat natural to Oskam, the original device’s control system did not: To initiate stepping movements, the system watched for small contractions Oskam was still able to make about his right hip and then moved his legs accordingly.
The addition of the brain-computer interface to complete the BSI system makes walking controls much more intuitive. At a press conference, Oskam commented on the improvement stating that in the old system, “the stimulation was controlling me. Now I’m controlling the stimulation.”
Another benefit provided by the BSI is a degree of repair to the damaged tissue in Oskam’s spine. After learning to use the stimulation system over a period of weeks and months, Oskam and the researchers found that he was regaining the ability to move his legs without the device’s help. They first saw this recovery as Oskam learned to use their initial hip-driven stimulator system, but saw substantially greater healing of the spine with the addition of brain-driven control.
This recovery, according to the research team, is driven by the device reestablishing the coordinated behavior of neurons both transmitting into and reading out from the damaged section of the spine. When proximally located neurons reliably activate at similar times, our nervous systems tend to connect them. In Oskam’s case, the BSI system stimulated neurons in the spine below the injury but neurons above the damaged tissue were still firing because they are still connected to the parts of his brain initiating and controlling leg movement. “The detailed mechanism we don’t know, but the whole idea is that the sensorimotor loop is closed,” says Courtine. “He’s trying to activate a region with his natural pathways that are also stimulated at the same time. This cooperation between the natural and digital pathways is likely driving regrowth of the projections.”
The researchers see this result as a promising proof of concept, and are looking to expand their research to help more patients with spinal injuries and have partnered with device company Onward Medical to miniaturize their BSI system for clinical applications. Courtine and his team are also exploring other uses for their BSI system, including potentially restoring lost arm movement to tetraplegic spinal injury patients. “There is no reason why the same principle could not be applied for the recovery of arm and hand functions,” says Courtine. “We have obtained approval to test this digital bridge for the recovery of arm and hand function in humans and are recruiting patients.”
- One Small Step for a Paraplegic, One Big Step Toward Reversing Paralysis ›
- Fixing the Brain-Computer Interface - IEEE Spectrum ›
- Exosuit Muscle Control Steps Closer to Reality - IEEE Spectrum ›
Michael Nolan is a writer and reporter covering developments in neuroscience, neurotechnology, biometric systems and data privacy. Before that, he spent nearly a decade wrangling biomedical data for a number of labs in academia and industry. Before that he received a masters degree in electrical engineering from the University of Rochester.



