Next-Gen Ultrasound
Medical imaging borrows techniques from the microelectronics industry
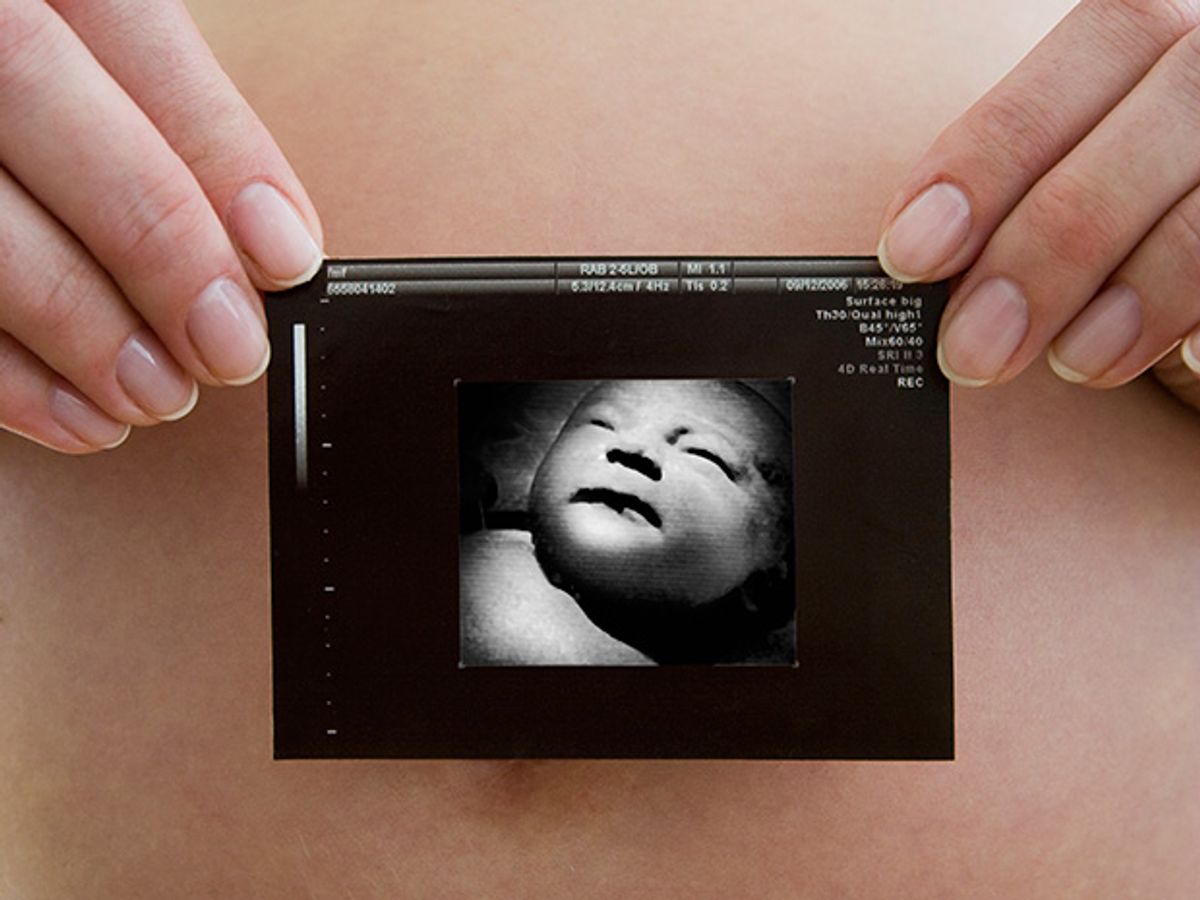
Almost invariably , a new baby’s photo album begins with a grainy black-and-white picture taken months before birth—a prenatal ultrasound image, which is often detailed enough to inspire comments about the child’s resemblance to various members of the family. But jokes about balding uncles notwithstanding, such scans serve a serious purpose and can prove immensely important, as when they allow doctors to diagnose and sometimes even repair a congenital malformation while the baby is still in the womb.
When seeing such an image for the first time, most people are awestruck. How can mere sound waves provide such remarkably clear views? Engineers may well ask something more: How can we give doctors even better ultrasound images? That question has engaged the three of us, along with other members of our Stanford acoustics group, for much of the last decade.
Whereas the signal-processing and image-reconstruction techniques used in medical ultrasonography have made huge advances since this type of imaging became commonplace three decades ago, the business end of the apparatus—the transducer, which converts electrical impulses to sound waves and vice versa—has remained largely unchanged. So we found fertile ground when we began digging for ways to improve those transducers using tools from the microelectronics industry. You will soon find the fruits of those efforts at your local hospital. Indeed, this strategy promises to revolutionize ultrasound imaging within the next few years.
How ultrasound imaging works is easy enough to describe, at least in broad strokes. High-frequency (1- to 50-megahertz) sound waves transmitted into the body create reflections when they encounter a change in tissue density or stiffness. These faint echoes are picked up with the same set of transducers used to generate the sound. Or the imager may use just a single transducer moved over the body—usually with the aid of much slimy goo, to ensure good acoustic coupling. The resulting electrical signals are then amplified, combined, and displayed as images.
Ultrasonography is valuable for several reasons. For one, it’s inexpensive—at least compared with CT (computed tomography) and PET (positron-emission tomography) scanning, or with MRI (magnetic resonance imaging). Also, the low-amplitude ultrasound waves used for imaging do not involve ionizing radiation and are thus harmless to the patient, so repeated scans can be made without worry. And with this technique it is not difficult to get real-time imagery, which doctors may want for such things as guiding a biopsy needle. These virtues make the market for medical ultrasound equipment huge—more than US $5 billion annually, a figure that’s only expected to swell in coming years with growing sales of these systems in China and India.
An ultrasound imager has four main parts: the transducer probe, the analog front-end electronics, the digital signal-processing hardware, and the display. Advances in electronics over the years have brought an extraordinary level of refinement to all but the transducer, which means that most of the remaining opportunities for improving system performance lie in the design of this one critical component. In particular, researchers have lately been seeking reliable ways to fashion many individual transducers into compact arrays.
Having a series of transducers laid out in a line—a one-dimensional array—is the simplest example of this strategy. Such transducer arrays are now employed routinely for most forms of ultrasound imaging. Like multielement radio antennas, such arrays can be steered so as to send energy in a narrow, directed beam. Steering an array also works in reverse, allowing it to detect acoustic echoes that come from one particular direction. While a one-dimensional transducer array can be steered and focused within a single plane to make a two-dimensional image, a 2-D array can be steered and focused throughout a volume to make a three-dimensional image—and this can be done in real time.
With this capability, physicians can, for example, follow heart motions in great detail if they want to assess a patient’s cardiac functioning. In the not-so-distant future, such ultrasound imaging may even allow robotic surgeons to operate on a beating heart so that patients need not run the risk of having to depend on a heart-lung machine.
In the nearer term, doctors are keen to use small 2-D arrays of tiny ultrasonic transducers to obtain forward-looking images as they probe an artery with a catheter. That would permit them to examine obstructions and map the composition of plaque deposits on vessel walls in three dimensions. What’s more, sufficiently small transducers can be arranged in a ring on the end of a catheter, leaving space at the center for an excision device. Such an instrument would allow for simultaneous ultrasound imaging and surgical therapy.
Two-dimensional arrays of ultrasonic transducers would certainly help physicians perform minimally invasive treatments in this way. But making such tiny arrays using traditional transducers is frustratingly difficult. Fortunately, the precise fabrication required can readily be carried out using methods developed by the microelectronics industry, methods that are now routinely used to produce various sorts of microelectromechanical systems, or MEMS.
MEMS fabrication techniques have enabled us to construct something we call a capacitive micromachined ultrasonic transducer. This name, we admit, is an ungainly mouthful, and the acronym we use in our scholarly papers, CMUT, is a bit cryptic to all but a few specialists. Perhaps this is why some of our colleagues in industry refer to this new technology by the more pleasing phrase “silicon ultrasound,” which tells you right away what stuff these new transducers are for the most part made of.
Ultrasonic transducers have traditionally been fashioned from piezoelectric materials like quartz or lead zirconate titanate, which many engineers know as just PZT. These are crystals or ceramics that expand or contract in response to an applied voltage. Likewise, piezoelectrics will generate an electric signal in response to being stretched or squeezed, so they can both transmit sound and detect it. This is very old technology, having been invented late in the 19th century, when the brothers Pierre and Jacques Curie demonstrated piezoelectric effects in what for decades remained a laboratory curiosity. The first real application, for sonar, came in 1917.
The procedure used to process a piezoelectric substance into a transducer or an array of transducers relies mostly on age-old manufacturing methods: mixing materials, bonding them, mechanically dicing the resulting assembly, adding wiring—that is, a lot of delicate manual labor. The production of ultrasonic transducer probes, which amounts to a global market of about $1 billion annually, is therefore limited by the many headaches involved in maintaining high yield and good product uniformity in a manufacturing system that depends so much on sharp eyes and steady fingers.
The capacitive transducers we’ve been pioneering sidestep such issues. By using photolithography and other fabrication techniques of the semiconductor industry, we can make transducer arrays—large or small—with even the most complex geometries, and we can do so very precisely and inexpensively.
In a way, it’s surprising that this approach has taken so long to catch on. After all, condenser microphones are capacitive sound transducers, and they’ve been common for decades. They change sound into electrical signals using a flexible membrane separated from a solid back plate by a very thin air gap. Both membrane and back plate are conductive, or have conductive electrodes attached to them, so a condenser mic is essentially a parallel-plate capacitor. When sound waves hit the membrane, it vibrates, inducing an oscillatory current from the capacitor when it is biased with a dc voltage.
Condenser microphones can capture sound of superb quality, which is why they are often used in studio recording. In ultrasonics, though, the demands are greater than they are for audio frequencies. For a capacitive design to be as efficient as the existing piezoelectric transducers, the electric field in the gap has to be enormous—hundreds of millions of volts per meter. And when subjected to electric fields of that magnitude, air tends to break down, forming a conductive arc. So if you tried running a normal condenser microphone with a bias voltage high enough to produce such an electric field, you’d soon see sparks fly.
Fortunately, the world works differently at small scales. As you reduce the size of the gap, the electric field required for air to break down increases. So with a sufficiently small gap, you can make a capacitive transducer—essentially, a tiny condenser mic—that supports an immense electric field. Such a transducer can be extremely efficient.
That a small gap can sustain a large electric field has been known for more than a century, but it wasn’t until the early 1990s that a few researchers took advantage of this fact and began experimenting with capacitive transducers for ultrasonics. They struggled, though, using mostly conventional machining and plastic-film membranes (in a few cases with micromachined back plates) and were unable to overcome the breakdown issue in their first crude devices. Then in 1994 one of us (Khuri-Yakub) and Matthew I. Haller, who was at that point a graduate student in our research group at Stanford, began to apply micromachining and other MEMS techniques to construct the entire transducer.
At the time, our focus was on equipment for nondestructive testing—looking for cracks in the wings of F-18 fighter jets, to be specific. So we intended these first transducers to be used in air (where they worked surprisingly well). Having previously done a lot of research for the U.S. Navy on sonar, though, we tried out a pair of the new transducers underwater for kicks. What we saw knocked us off our chairs. When immersed, the new transducers displayed phenomenal bandwidth, much better than piezoelectrics. Actually, they showed this stellar performance for all of 15 minutes or so; then they stopped functioning altogether.
After a certain amount of cursing and head scratching, we figured out the reason. In our initial designs, the gap between the membrane and the substrate was left open to the outside environment. That’s fine for use in air, but when these transducers were immersed, water slowly made its way into the gap, ruining their ability to operate. But it didn’t take us long to figure out how to seal these cavities. And eventually we devised ways to eliminate the air inside altogether.
Further work also showed why these capacitive transducers have greater bandwidth than piezoelectrics. The difference arises because a piezoelectric transducer is by nature a highly tuned device, like the pendulum of a clock. At its particular resonant frequency, a piezoelectric transducer undergoes high-amplitude oscillations, even with very little forcing, but at other frequencies, it barely moves at all—which is to say that it has very limited bandwidth.
A capacitive transducer also has a distinct resonant frequency, but only when it’s operating in air. When it’s immersed in water—or coupled to biological tissues, which are much like water in their acoustic properties—the situation becomes very different. Because the vibrating membrane has so little mass, its movements become highly damped by the watery medium it touches. The same thing happens if you place a pendulum under water. It’ll no longer oscillate at its normal resonant frequency, but it can still swing back and forth at the frequency you’re using to drive it. This effect, then, lets a single transducer work well over a broad swath of the ultrasonic spectrum.
That’s important because it means that the transducer is able to emit and detect the many different frequencies that are contained in a short ultrasonic pulse. The shorter the pulse you use to probe the patient’s body, of course, the better the depth resolution in the resulting image. And improved resolution is, after all, just what the doctor ordered.
We construct one of our transducers by connecting many small units in parallel. Each contains a thin membrane, separated from an underlying substrate by a tiny gap. In our latest designs, the membrane is made of silicon, possibly covered with a metal electrode. Silicon is desirable for several reasons. One is that it has good mechanical properties—it doesn’t fatigue, for example—and as long as it is thin, it will flex sufficiently. The substrate is silicon as well, doped with a sprinkling of other atoms to make it highly conductive.
We’ve developed different recipes for making these devices over the years, but the best scheme we’ve found uses two different wafers: a garden-variety silicon wafer for the substrate and one that’s slightly more exotic for the membrane, something known in the semiconductor industry as silicon-on-insulator. The two are bonded together using nothing more than a modest amount of pressure and heat. This two-wafer approach permits us to add the membrane after the pockets that serve as the gaps are already formed, so we can sculpt the membrane and substrate as we wish—they don’t have to be just flat planes.
Building transducers from silicon makes it a snap to connect them with the front-end electronics of an ultrasound imager. Although it’s possible to fabricate a transducer directly over the associated electronic components on the very same silicon wafer, doing so creates a number of troublesome complications. The better tactic, we’ve found, is to bond the finished transducer array to a separate wafer containing the electronic circuitry.
Connecting each transducer element may be tricky for tightly packed 2-D arrays, because there isn’t much free real estate on the front surface to route a lot of electrical leads. But here again the microelectronics industry has a good solution: Make the connections to the electronics by burrowing down through the transducer substrate and creating vertical conductive channels, which are known in the trade as through-silicon vias.
Given the many wonderful things we’ve said about them, you might think that capacitive micromachined ultrasonic transducers would already be in use in medical imaging equipment. Many of the companies that make these systems have indeed embraced this technology, but it hasn’t yet reached vendors’ shelves. Most of the remaining technical issues are minor, though. Some stem from the electric fields these transducers must contain.
Although there is no chance of arcing across the evacuated gaps, the enormous electric fields can stress the insulating layers to the breakdown point. And even without that, these large fields can inject static electric charge into those layers, which reduces the electric field in the gap, making it necessary to keep adjusting the dc bias field to compensate.
Another challenge with capacitive transducers is that they do not respond as linearly to drive voltages as PZT transducers do. Nonlinearity of the transducer becomes an issue when an ultrasound system is used to image the nonlinear response of biological tissues. Fortunately, there are ways to circumvent this problem, such as purposefully distorting the drive signal to compensate for the nonlinearity of the transducer.
We—along with a slew of engineers at Canon, General Electric, LG Electronics, National Semiconductor, Siemens, and elsewhereare working to solve these nagging problems and to confront the many other practical realities you have to deal with in any new product. That’ll take some time, but it’s clear to us that there are no showstoppers here.
It won’t be long before this new breed of transducers arrives at hospitals all over the world. So expect those first baby pictures you’re shown, among other sorts of ultrasound images, soon to become even more stunning.
About the Author
Butrus T. Khuri-Yakub, Ömer Oralkan, and Mario Kupnik can all put “Dr.” in front of their names, although you can’t turn to them for a prescription. Still, the work they describe in “Next-Gen Ultrasound” brings them nearly as often to Stanford University’s medical school as to its engineering school. Khuri-Yakub, a professor of electrical engineering, manages technical operations for Stanford’s E.L. Ginzton Laboratory, where Oralkan is a senior scientist and Kupnik is a research associate.
To Probe Further
A detailed summary of the authors’ MEMS-fabrication techniques is published in “Capacitive Micromachined Ultrasonic Transducers: Fabrication Technology,” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control , vol. 52, no. 12, December 2005.
The full range of the authors’ research in acoustics is described at https://www-kyg.stanford.edu.