Almost a decade ago, Stanford University researchers jump-started a new field of study when they genetically altered brain cells, shone light on them, and demonstrated that they could control the modified neurons. This brain-control technique, called optogenetics, has since taken off at labs around the world and could lead to breakthroughs for Parkinson’s disease, addiction, depression, and spinal-cord injuries.
But optogenetics uses complicated processes that require the separate delivery of genes, light, and electricity to a tiny part of an animal brain. Now researchers in Germany and Switzerland have found a way to simplify the procedure by building a flexible implantable device that delivers all three things. The researchers recently reported their results in the journal Lab on a Chip.
In optogenetics, scientists insert genes for light-sensitive proteins found in algae into mammalian nerve cells. The genetically modified neurons then begin to produce those proteins. Shining light of one color on the neurons causes the proteins to open certain channels in the cell membrane, letting in sodium ions that activate the neuron. Light of another color causes an influx of chloride ions, turning the neuron off. This provides precise control and is a leap forward from electrical stimulation, which is usually used to trigger a response in the brain. While electrical stimulation excites all the nerve cells around a relatively large electrode, light can trigger the specific neurons that took up the new genes and can switch them on and off in milliseconds, the timescale at which neurons fire.
Optogenetics methods are cumbersome. First, researchers inject gene-carrying fluids, or vectors, into a spot in the brain using a syringe. Six weeks later, they try to maneuver the end of a thin silicon waveguide to the same spot to direct light to the altered neurons. “If you’re a lucky person, you are at the same point where you injected the vector,” says Thomas Stieglitz, a professor in the department of microsystems engineering at the University of Freiburg, in Germany, and a member of the team. “But then you have to implant a third thing—an electrical probe—to record signals.”
Getting to the same cubic millimeter of the brain three times is exceedingly difficult. So some research groups have recently begun making “optrodes,” which are made up of light waveguides and electrical probes. The optrodes either fix an optical fiber onto the probe—both are made of silicon—or coat the silicon probe with a waveguide made of a photoresist material.
The German and Swiss researchers went one step further. They integrated an optrode with a microfluidic channel that can deliver gene vectors. The result is an implant that performs all three essential optogenetics procedures: delivering gene-carrying fluids, stimulating neurons with light, and recording the brain cells’ electrical response. Additionally, says Stieglitz, the new polymer device is more flexible and biocompatible than the silicon tools used for optogenetics today. And because it does not interact with light, it should not interfere with the optogenetic process.
The new integrated implant is a “major leap forward,” says Polina Anikeeva, a materials science and engineering professor at MIT who was not involved in the research. “The elegant integrated design will undoubtedly open a number of exciting basic neuroscience experiments.”
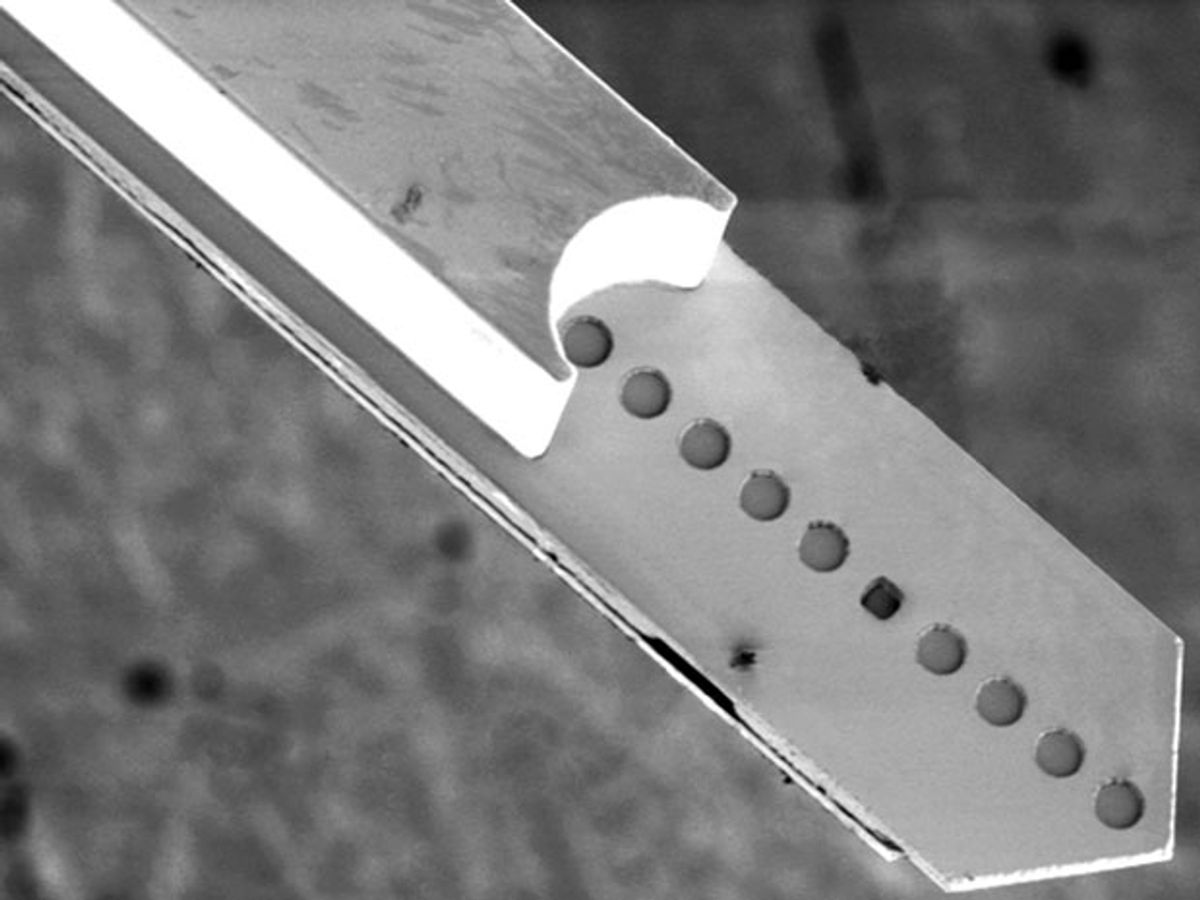
Stieglitz and his collaborators at the Friedrich Miescher Institute for Biomedical Research, in Basel, Switzerland, started by creating small platinum electrodes on the pointed tip of a flat polymer probe. They then added a coating of photoresist material that acted as a waveguide. Separately, they made a channel out of the same photoresist material and glued it to the underside of the electrode-carrying polymer shaft. A small hole in the polymer probe served as the channel’s output.
The design puts all three crucial features—the opening of the gene-delivering microfluidic channel, the end of the light-guiding waveguide, and the signal-recording electrodes—at the probe tip, so targeting neurons in a tiny portion of the brain is no longer a matter of chance.
The probe is inserted in a single procedure and left there for the six weeks or longer that it takes to conduct an optogenetics study, Stieglitz says. Tests in live, unconscious rats showed that the implant could turn neurons on and off as expected. The researchers are now doing more-detailed studies, including testing on conscious animals, to see if they can induce behavior changes.
Right now, optical fiber and electric wire exit from the subject’s skull. But the German-Swiss team says it is trying to integrate the prototype implant with a light source and an electrical circuit that could make the device wireless and completely implanted. Eventually, says Stieglitz, they want to make the device with a degradable polymer “that vanishes over time.”
About the Author
Prachi Patel is a contributing editor to IEEE Spectrum. In January 2013, she reported on how metamaterials would allow for much smaller millimeter-wave scanners.
Prachi Patel is a freelance journalist based in Pittsburgh. She writes about energy, biotechnology, materials science, nanotechnology, and computing.