Bringing HIV Labs by Backpack to Rural Africa
Diagnostic medicine goes mobile in Africa, thanks to new microfluidic tools
When John Barber, a project manager at Daktari Diagnostics, sought to test his company’s instrument, he went to the type of place where the technology might have the most impact: a small fishing village on the shores of Lake Victoria in Uganda. He awoke at dawn on a November morning in 2013, tossed a few Daktari devices into a backpack, and, together with a team of HIV-treatment specialists, drove 2 hours to the village of Kasensero, where the first Ugandan case of HIV was reported more than 30 years ago. Driving a Jeep along dirt roads with more cows than traffic, “we were off the grid,” Barber recalls.
Barber and his team showed up at 8 a.m. and found about 20 people already waiting for them. Dozens more arrived within the hour. An estimated 43 percent of people in Kasensero are HIV-positive, and these patients wanted to know whether the virus had started to damage their immune systems. The medical team was there to check the patients’ CD4 counts, a measure of immune cells that indicates how well the body can stave off opportunistic infections such as tuberculosis. Based on test results, some people would need to start antiretroviral therapy. Others might need their medications adjusted.
The villagers didn’t get answers that day. While the Daktari device is capable of providing same-day results, it was only being tested during Barber’s visit to Uganda. So the Kasensero patients had to settle for the standard CD4 diagnostic procedure: testing on a large and expensive desktop instrument called a flow cytometer, which requires dedicated laboratories, highly trained technicians, and infrastructure for shipping refrigerated reagents long distances. Because the nearest flow cytometer was in a town 80 kilometers away, the HIV-treatment team had to collect and transport vials of blood for testing and couldn’t inform villagers of their CD4 levels until they returned for their next scheduled visit months later. At worst, patients were lost to follow-up. At best, critical medical decisions got delayed. Either way, the virus became tougher to fight.
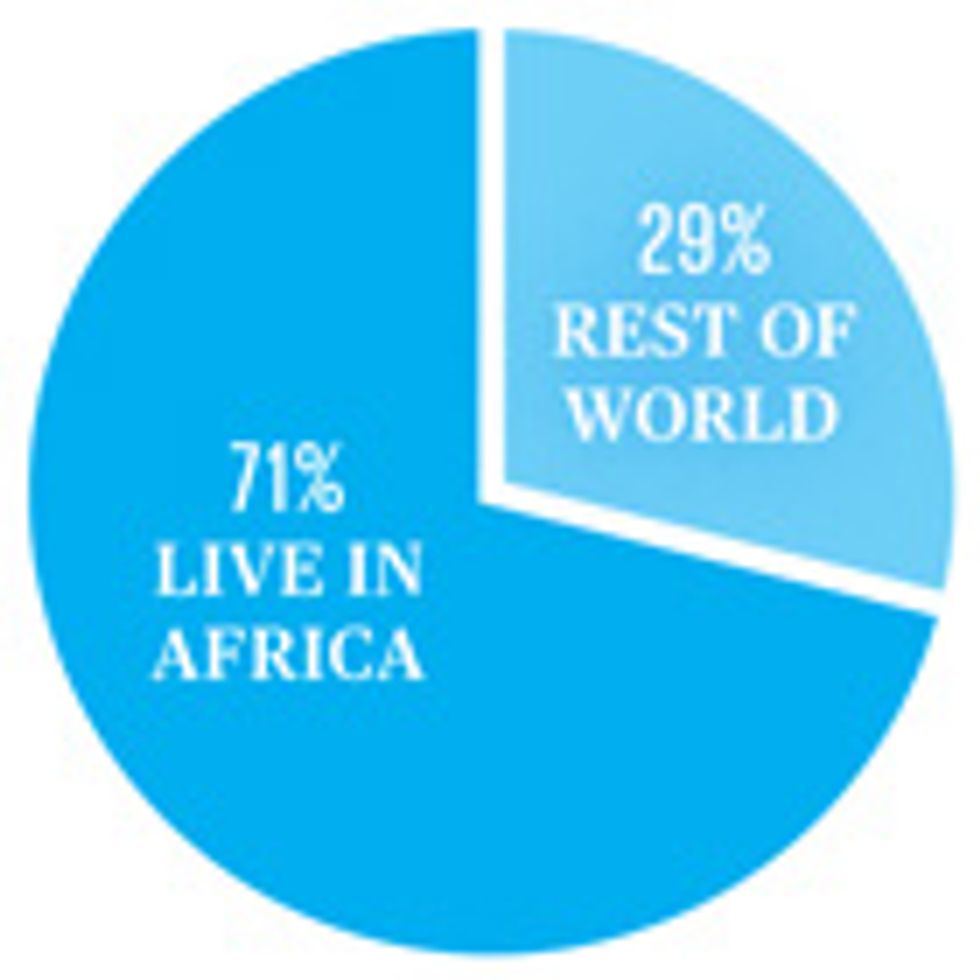
People living with HIV
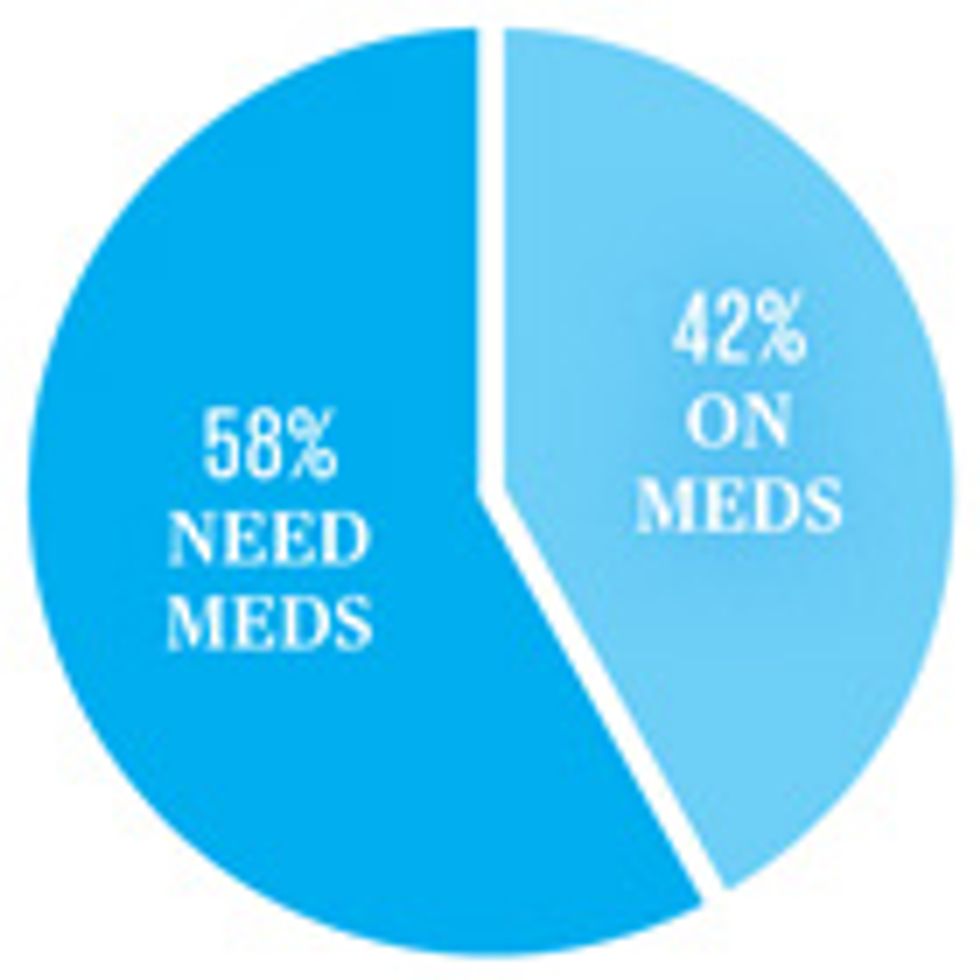
HIV medication in Africa
The World Health Organization recommends that adults with HIV begin taking antiretroviral medications when their CD4 cell counts fall to 500 cells per cubic millimeter.
In 2015, Daktari will enter the fray. The company is planning to roll out its portable CD4 tester this year across sub-Saharan Africa—first in Kenya, then in Ethiopia, South Africa, and practically every country in between. The Daktari device is part of a new wave of lab-in-a-backpack instruments that can bring diagnostic testing directly to patients and health workers in the developing world.
The device looks like a vintage Fisher-Price tape player, with test cartridges taking the place of audiocassettes. But that appearance belies a sophisticated piece of equipment that can provide CD4 counts in less than 15 minutes, all from a simple finger prick. “Our goal is that you have a CD4 count and you can make a decision before the patient leaves the doctor’s office,” says Aaron Oppenheimer, vice president of product design and development at Daktari, as he demonstrates the instrument at the company’s headquarters in Cambridge, Mass. “There really aren’t products like this on the market. In fact, there really isn’t a market. There’s a need, but being able to do real diagnostics at the point of care is new.”
Oppenheimer acknowledges that a few rival companies already have grab-and-go tests available for CD4 monitoring in rural Africa. But these competing devices are essentially portable versions of flow cytometers, which rely on imaging fluorescent proteins bound to CD4 cells. Daktari’s CD4 reader instead measures the electrochemical signal of the cells, and Oppenheimer says this distinction is important. Without optical detection, he says, the Daktari device has a longer battery life—up to two days or about 50 runs—and requires less maintenance than other systems. “We believe that our product will go where other products that we’re competing with can’t go,” Oppenheimer says.
Doctors have long talked of the promise of point-of-care diagnostic tools that can provide near-instant results at hospital bedsides and doctors’ offices. In recent years, that talk has turned into real products. Most of these portable devices rely on new microfluidic technologies, which manage how tiny samples of bodily fluid move through pieces of equipment. They also make use of miniaturized electronics developed for cellphones and other consumer electronics. According to a recent report on microfluidic diagnostics by Yole Développement, a consulting firm in France, the worldwide market will grow rapidly: from US $1.6 billion in 2013 to a projected $5.6 billion in 2019.
It’s in global health that point-of-care diagnostics could arguably do the most good. In countries like Uganda, where many people don’t have access to hospitals and labs, these tools could empower a distributed, mobile system of health-care delivery. Just as African telecommunications largely skipped over landline infrastructure and went straight to mobile phones, some experts say African medicine can skip over centralized labs.

Device makers are working on a wide range of disease applications, with HIV-management tests at the forefront. In high-income countries, doctors routinely check the amount of virus present in an HIV patient’s blood (in addition to counting CD4 cells) to guide treatment decisions, such as when to switch from one drug to another. But costs and equipment demands have precluded running viral-load tests in most of the developing world. “The existing systems are not anywhere near good enough to do the job” in resource-limited settings, says Daniel Laser, CEO and president of the diagnostics company Wave 80 Biosciences, in San Francisco.
For newborn babies, the viral-load count is critical. Doctors can diagnose HIV in adults and older children with simple flow tests that identify the presence of antibodies directed against HIV, but these same antibodies diffuse across the placenta and can persist for up to 15 months in an infant’s blood. Thus, their presence confirms that a mother is HIV-positive but doesn’t reveal a newborn’s HIV status.
A number of companies are now introducing devices that can check viral load in this vulnerable population. Wave 80, for example, has invented a handheld device called the Eoscape-HIV, which uses disposable cartridges that incorporate micropistons. It can measure viral loads on-site in less than an hour. The Massachusetts-based company Alere also has a new viral-load-testing device, which it’s launching in late 2015. “The clinical reality is, if we don’t get a [HIV-positive] baby on treatment within the first 8 to 12 weeks of life, the mortality is quite bad,” says Willem Pretorius, global product manager for HIV care at Alere. “If we bring a point-of-care early-infant diagnostic test to the primary health-care setting, where the mother can actually wait for the result, that could have quite a big impact on getting those babies who test positive on antiretroviral therapies.”
Many of these same HIV-monitoring technologies can be refashioned for other diagnostic purposes. Wave 80, for example, is looking also to detect chlamydia, gonorrhea, and other pathogens with its Eoscape. And Alere is adapting its new machine to test for tuberculosis, including drug-resistant strains. These portable systems may soon allow health-care workers to test for many infectious diseases in the remotest regions of the globe.
In 2015, “the bottleneck is not really in the technology” anymore, says Benjamin Roussel, an analyst with Yole. “It’s mainly in the user, the doctor, and the medical world. Getting them to adopt the technology could take some time.” Fortunately, that adoption process has begun.
Fast Forward
Stripping Away the Instruments
Paper-based tests and cellphones will replace dedicated devices
By 2020 To make diagnostics even easier in the developing world, some innovators are creating tools that require no maintenance and no power source.
A forthcoming CD4-counting tool from Scotland-based Omega Diagnostics, for example, takes the same approach used in a pregnancy test, wicking blood through paper and capturing proteins from CD4 cells at a sample line. This is checked against a reference line to give a visual “treat or no-treat” result, explains the device’s inventor, David Anderson. “It can be read by eye,” he says. “You don’t need anything except the disposable that comes in the box to get a result.”
Omega has also created an app to allow more precise quantification with a smartphone camera. Other companies are coupling cellphones with paper-based microfluidics to detect levels of blood sugars, micronutrients, and pathogenic bacteria. “Cellphones are ubiquitous,” says Elain Fu, a bioengineer at Oregon State University, so there’s little sense in building a dedicated read-out tool for every test. Within five years, Fu expects most paper-based microfluidic tests to be read by smartphones.
By 2025 At the nonprofit Diagnostics for All, based in Cambridge, Mass., CEO Marcus Lovell Smith is looking further ahead. His team is blending disposable electronics, including LEDs, light detectors, and transistors, into paper-based microfluidic tests. “We are absolutely averse to instruments because we feel that anything that plugs into a wall, or requires some sort of calibration or servicing, is just not feasible,” he says. “But that doesn’t mean that we wouldn’t use sophisticated but very inexpensive electronics.” Those hybrid systems are maybe a decade from commercialization.
Bernhard Weigl, who works on diagnostic technology at Path, a Seattle-based nonprofit, says scientifically sophisticated paper tests could be the world-changing advance the global health community has been waiting for. This is “quantum-leap-type stuff,” he says. —E.D.
About the Author
Elie Dolgin is a freelance science journalist based in Somerville, Mass. Before moving into journalism, Dolgin earned a Ph.D. in evolutionary genetics, studying the development of sex in the Caenorhabditis elegans worm. He now writes about human health and biomedical research, and he’s thankful for his years in the lab, which he says taught him to “judge good science.”
This article originally appeared in print as “Portable Pathology for Africa.”