This Maglev Heart Could Keep Cardiac Patients Alive
Inside Bivacor’s artificial heart, a levitating disk spins 2000 times per minute to keep blood flowing


For more than 50 years, cardiac surgeons and biomedical engineers at the Texas Heart Institute (THI) have been questing for an artificial heart that can fully replace natural ones, which are in terribly short supply for transplant. They’ve seen their share of metal and plastic contraptions that used a variety of pumping mechanisms, but none of these machines could match the astounding performance of the human heart.

In April 2019, the possible culmination of that long quest was inside a shaggy brown cow, which stood peacefully chewing its cud at a THI research facility in Houston. The animal was part of a 90-day trial in which it lived its life powered by an implanted artificial heart made by our company, Bivacor. Throughout the trial, the calf stayed healthy and energetic, and gained weight at a normal rate. It even jogged on a treadmill for 30-minute stretches.
Our company is now working toward human trials of our device. It relies on a dramatic new approach: Rather than using a mechanical pump that mimics the structure and actions of the four-chambered human heart, it uses a spinning disk, suspended in a magnetic field. With just one moving part, the Bivacor heart is able to send oxygen-rich blood out to the body and return oxygen-depleted blood to the lungs.
We had to overcome many technical challenges to make an artificial heart that’s small, biocompatible, energy efficient, and durable. Consider that the human heart beats about 112,000 times a day, which adds up to 42 million times a year, and you’ll understand the magnitude of the challenge.
We’ve tested the Bivacor heart in 15 cows so far. While the need for animal testing is unfortunate, it’s the only way to prove the device’s safety and move forward to clinical trials in humans. These Corriente calves, which are relatively small, are the right size to serve as analogues for adult patients. We’ve also implanted the Bivacor heart in several sheep, which are more representative of patients with smaller bodies, including children. Our tests have shown that the heart holds up well: With its one moving part levitating in a magnetic field, there’s no worry that friction and mechanical wear will cause the machine to give out. Our tests have also shown that the device can adapt to the user’s cardiovascular requirements.
One of us, Bivacor founder Timms, began working on this project 18 years ago. [For more about his personal story, see "Artificial Heart Inventor Was Inspired by His Plumber Father."] Through the years, the central concept of the maglev heart has stayed the same, though our engineering team has greatly refined the technology. We’re thrilled that the device is nearly ready for human trials because we think the Bivacor heart is the solution that severely ill heart-failure patients have long been waiting for.
In heart failure, the heart becomes unable to pump enough blood to keep the body healthy and strong. At least 26 million people around the world are living with the disease, and the number is rising as populations age. Patients with severe heart failure have a bleak outlook: Their best option is a heart transplant, but the limited number of donor hearts means that only about 5,000 patients around the world receive transplants each year. Thousands more patients are eligible for transplants, and some die while waiting for a donor organ.

Cardiologists have long dreamed of a mechanical replacement. In 1969, THI physician Denton Cooley implanted the first “total artificial heart” in a patient who was awaiting a transplant, keeping him alive for the 64 hours it took for a donor organ to arrive. However, that patient died shortly after the transplant surgery from an infection, and Cooley’s team shelved the device due to concerns about reliability and compatibility with the human body.

Since then, a handful of other total artificial hearts have been developed, and a few have made it into human trials. But these devices were large, heavy, and prone to mechanical failure, and only two gained regulatory approval in the United States. The SynCardia Systems artificial heart was approved in 2004 as a “bridge to transplant” and is currently being tested as a permanent replacement. However, users must carry a 6-kilogram case containing a loud air compressor that’s attached to tubes penetrating the abdomen, where the air drives the device’s two pneumatic pumps. A second artificial heart, the AbioCor, gained approval in 2006, but it was discontinued almost immediately, when the company behind the device decided it wasn’t commercially viable.
The most common mechanical support now in use is what’s called a left ventricular assist device (LVAD). This machine adds its pumping power to an ailing heart, focusing on the left ventricle, which pumps oxygen-rich blood through branching arteries that reach up to the brain and down to the toes. LVADs are now being used as both temporary aids for patients awaiting transplants and, in cases where transplantation isn’t an option, as permanent additions to patients’ chest cavities. However, LVADs can cause the right ventricle to falter, requiring intensive drug treatments and sometimes the implantation of a right ventricular assist device.
At Bivacor, we wanted a true long-term mechanical replacement for the entire heart. Unlike most earlier attempts, our effort didn’t set out to mimic the heart’s natural pulsatile pumping mechanism, with valves that open and close as the left ventricle pushes blood out to the body and the right ventricle pushes blood to the lungs. Most prior artificial hearts used positive displacement pumps to achieve this effect: Their two artificial chambers were bifurcated with membranes that flexed forward to push blood out through mechanical valves.
We opted instead for a centrifugal pump that propels a continuous stream of blood into the arteries. Such a pump has no valves, so a patient using the Bivacor heart in its most basic mode would have no pulse. But we recently adapted our device to give it a pulsatile outflow option, as we’ll explain below. We want clinicians to look at the cardiac monitors for patients with implanted Bivacor hearts and see the familiar rhythmic readouts they’ve seen since medical school.
The Bivacor heart would fit in the palm of your hand—it’s about 650 grams, slightly heavier than an adult human heart. Its shell is made of titanium, a noncorroding material that almost never triggers an immune response. Patients will wear a 4-kg external controller pack that contains two rechargeable batteries (providing about 5 hours of operation each), although they can also plug in directly to a power outlet.
Timms has been developing his design for an artificial heart since 2001. The first concept model tested the hydraulic feasibility of the design, with a single rotor pumping fluid in two directions.




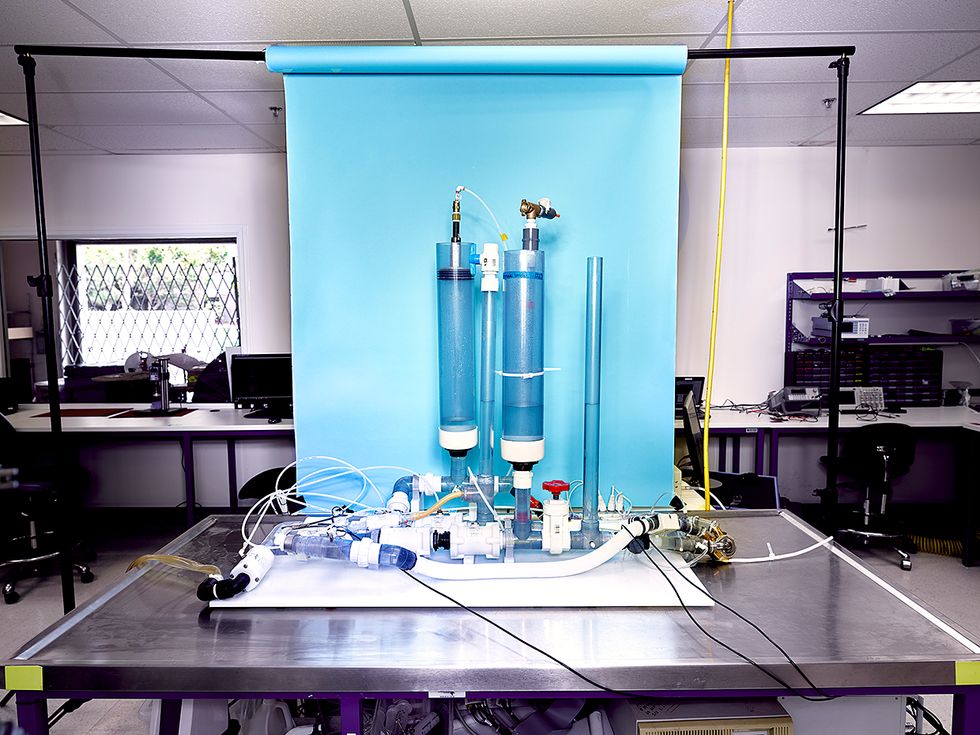
Throughout our design process, we used 3D printers to make both titanium and plastic parts for our prototypes, allowing us to rapidly experiment with different geometries. For testing, we built a hardware simulation of the human circulatory system in our engineering office in Los Angeles; this mock-up allows us to validate a device’s function thoroughly and repeatedly in a controlled environment, and reduces the need for animal testing.
The primary design innovation in the Bivacor heart is its simple construction, with one motor and one rotating disk that simultaneously supports the pumping of blood to both the body and the lungs. The rotating disk is completely suspended in a magnetic field, operating under the same “maglev” principle that has been used by high-speed trains. The disk has open impeller vanes on both sides, one larger set that pumps blood at the high pressure necessary to send blood throughout the body, and another smaller set that pumps blood at lower pressure to the lungs. Each side of the disk can pump more than 12 liters per minute, more than enough output for patients engaged in moderate exercise.
While some blood from the two sides mixes around the edges of the suspended disk, this isn’t a concern, because of the direction of the flow. Some oxygenated blood leaks from the high-pressure side to the low-pressure side, which means that some already-oxygenated blood returns to the lungs. And this leakage is actually a design feature, not a bug. The wash of blood around the disk cleans out the casing and ensures that there are no areas where stagnant blood can form into dangerous clots.
The motor’s stator provides rotational torque by coupling to a set of permanent magnets in the rotor disk. During normal operation, it spins the rotor at speeds of between 1,600 and 2,700 rotations per minute. The attractive forces between the motor and the rotor are counteracted by the magnetic bearing on the opposite side of the rotor, which actively controls the rotor’s position within the casing. This active control system is necessary because the rotor naturally moves around as the patient walks, climbs stairs, jumps, and generally goes about daily activities. It’s important to keep the rotor properly suspended and to prevent it from bumping into the sides of the casing, which could damage the components and smash blood cells.
This positional control system works as follows: Tiny contactless sensors send out magnetic fields that interact with the rotor, determining its exact location many times per second. If the rotor is moving in one direction or another, the control system puts electrical energy into electromagnetic coils within several actuators, causing them to cancel out that movement.
The design of the magnetic bearing actuators was critical, particularly because they had to be small, light, and energy efficient, yet strong enough to compensate for all the jiggles and joggles created by a person on the move. We used computer simulations of the magnetic field to optimize the design, experimenting with different materials and geometries to find the configuration that would provide sufficient force within a small space. Keeping our device small and light means that it will fit inside people with small bodies, including women and children.
To improve the efficiency of our artificial heart, we also integrated a “zero power” controller into the suspension system. This controller monitors the additional electromagnetic power used by the bearing as it responds to external forces acting on the rotor, then moves the rotor to a position where the permanent magnets of the magnetic bearing system can provide a balancing force. This system doesn’t produce instant adjustments—that’s the job done by the main stability controller—but it does reduce the amount of power used by the magnetic bearing when exposed to external forces.
To rapidly test their artificial heart prototypes, and to reduce the need for animal testing, the Bivacor team built an apparatus that replicates the human circulatory system, simulating the flow of blood through the body and the lungs.



A unique feature in the Bivacor heart is that the rotor can shift along its axis of rotation to change the amount of blood moved by the left and right sides of the pump. When the rotor moves toward the left side of the casing, it brings the impeller vanes closer to the casing wall. In this narrow space, most of the blood is whirled around by the vanes and little flows over the vanes’ tips, thus increasing the left pump’s efficiency and consequently its output of blood to the body. This feature is useful for quick adjustments necessary in transitions, such as when a patient stands up.
Our device is elegant in its cooperation with the human body, adapting automatically to a person’s activity levels. When the patient is exercising, the mechanical heart will pump more blood out to the muscles, just as a biological heart does. This adjustment is accomplished by attending to the body’s feedback: When a person starts to run on a treadmill, the hardworking muscles in the legs and elsewhere use up oxygenated blood faster and squeeze the deoxygenated blood back into the circulatory system. That increased blood flow into the Bivacor heart causes both sides of the pump to move more blood, without requiring an increase in rotor speed. This seemingly basic functionality is actually the result of careful optimization of the hydraulic design. We relied on our 3D printer to make numerous experimental prototypes with casings and impeller vanes of slightly different shapes.
Biocompatibility is one of the biggest challenges in our field, because the interactions between a mechanical device and biological systems are so complex. For example, the delicate blood cells and other blood components can be damaged by rough transit through a device. As a key design feature of the Bivacor heart, we ensured that the blood has plenty of clearance between the levitating rotor and the casing and conduits. All flow paths have clearance gaps of at least 240 micrometers during normal operation, which is more than 20 times the size of a red blood cell. This design reduces the shear forces to which the blood is exposed, and also ensures that there’s no blood stagnation within the casing.

The pump can run at constant speed, producing continuous blood flow at a constant pressure, and in our early experiments we concentrated on testing this “pulseless” mode. But it’s easy to change its speed, and our later experiments proved that controlled speed changes could produce a wide range of flow and pressure characteristics. Running the pump first at high speed (sending out more blood) and then at low speed (sending out less) creates something resembling a biological heart’s pulse; rapidly alternating these two speeds creates something that looks like a normal heartbeat.
We’re now working primarily in that pulsatile mode. Within cardiology, there’s an open debate about whether a pulse is necessary for good health. Some patients with implanted LVADs that produce continuous (and thus pulseless) flow experience medical problems such as gastrointestinal bleeding, yet it’s not clear whether their devices are the cause. We hope that our device, which can be used in either continuous flow or pulsatile mode, can contribute to the scientific investigation of this important topic.
Bivacor founder Timms began working on the original concept for his artificial heart 18 years ago, and it has undergone many iterations over the years. Timms began the project in his native Australia, and since those days, the development team has benefitted from a network of passionate collaborators in Germany, Japan, Taiwan, and the United States. During various stages of the project, the team relocated to international laboratories to make use of our collaborators’ expertise. Currently, our team is based in the United States (in Los Angeles and Houston) and Australia (in Melbourne and Brisbane), and we’re focused on turning our prototype into a commercial product.
We’re quite satisfied with the design of our device. Now we’re standardizing the production process: Our 3D-printing techniques were well suited for the development phase, when we wanted to test prototypes and iterate rapidly, but now we’re switching over to precision machining, which will give the device’s parts a smoother polish and more exact and consistent dimensions. These procedural changes will make larger-scale manufacturing possible. We’re also documenting every step of both the production and implantation process to prepare for our first clinical trials.
By the end of 2019, we’ll have a clinical-grade system. Then we’ll submit our request to the U.S. Food and Drug Administration for an early feasibility study in human patients, which we hope will commence in 2020.
We are already envisioning these first human trials. Gravely ill patients will go into the operating room with failing biological hearts beating feebly in their chests, and come out of surgery with smoothly functioning Bivacor artificial hearts whirring away. As the devices send powerful streams of oxygen-rich blood coursing through their bodies, we hope the patients will quickly find their strength returning. If these patients can rise from their hospital beds, hug their family members, and continue their lives for many years to come, we’ll have taken a great step forward in the long quest for a total artificial heart.
This article appears in the September 2019 print issue as “The Maglev Heart.”
About the Authors
Nicholas Greatrex, Matthias Kleinheyer, Frank Nestler, and Daniel Timms are with the artificial heart company Bivacor. Greatrex and Kleinheyer are senior electrical engineers, Nestler is an engineering consultant, and Timms is the founder, CEO, and CTO.
 Read the back story behind Timms’s inventionPhoto: Daniel Timms
Read the back story behind Timms’s inventionPhoto: Daniel Timms