The Body Shops
Part human, part machine, replacement organs may one day extend your life
Fourth in a series of reports on biomedical engineering innovations.
Meet Bob.
Bob might be your next-door neighbor. He’s an affable, 60-something retired engineer who took a buyout before the Internet bubble burst. He golfs, enjoys his grandchildren, and likes to travel with his wife of 35 years. The only problem is that he’s getting old—and he knows it. He’s bald on top, his skin is starting to sag in places, his eyesight isn’t what it used to be, and he sometimes can’t hear what his dinner companions are saying. Even worse, the diabetes that he was diagnosed with in his 40s is getting more difficult to control: his blood sugar seems like it is up one minute, down the next. And it seems as though all his friends have had at least one heart attack.
Bob is worried about getting old.
As an engineer, Bob knows that the body is just one big system that runs by chemical gradients and electrical impulses. So why is it so difficult to come up with replacement parts when bodies like his start to break down?
Luckily for Bob—and especially for those of us who are younger and can wait a few more years—engineers and doctors, mostly in the United States, have stepped up their search for ways to build replacement parts.
The current effort is focused on so-called biohybrid or bioartificial organs, which combine living cells with materials such as silicon and polymers. The hybrid organs get their structure from the inorganic material while relying on living tissue, grown from cadavers, animals, or, one day, from the patient’s own body, to do the complex tasks they do best, such as processing biochemicals and filtering blood. Although biohybrids are being tested outside the body and are at least five years away from reaching the market, ultimately they are being designed as implants—seamless replacement parts.
Construction of replacement organs got off to a rocky start, when the first two companies to come out with a product, living skin, went bankrupt nearly two years ago [see “Synthetic Skin,” IEEE Spectrum, December 2002]. But tissue engineers have gone back to the lab. And nowadays it’s a semiconductor lab.
A good portion of the newest innovations involve growing cells in specifically ordered arrays on chips made of semiconductor material. Electrical impulses pattern the cells as they would be in a natural organ and stimulate them appropriately so they develop into the needed tissues. Researchers have taken a page from the books of microelectromechanical systems (MEMS) developers and have built tiny systems using semiconductor fabrication techniques to address diseases that are now either marginally treatable or untreatable.
Many, if not most, of the new biohybrid research projects target some of the primary disorders of aging: kidney failure, emphysema, and heart disease—all among the top 10 killers of Americans older than 65. In 2001, heart disease alone killed one-third of everyone older than 65 who died in the United States. Kidney disease also takes a significant toll: more than 72 000 people in the United States die each year from end-stage renal disease. And current, stopgap therapies are costly: the 350 000 Americans who need kidney dialysis require a total annual expenditure of US $25 billion.
“The number one organ needed right now is the kidney,” says Anthony Atala, director of the new Institute for Regenerative Medicine at Wake Forest University Baptist Medical Center, in Winston-Salem, N.C. Roughly 15 000 kidney transplants are performed in the United States every year, but 58 000 Americans and about 40 000 Europeans are waiting for kidneys at any given time. (Global numbers are difficult to come by, because few countries keep such statistics.) The dire shortage of kidneys—and the relative simplicity of their blood-filtering function—makes them likely to be the first biohybrid organs available to patients.
Diabetes and hypertension are the top two reasons for needing a kidney transplant, and the organ-damaging effects of those two diseases escalate with age. There are two basic types of kidney failure. Acute failure is reversible and typically caused by a severe infection or as a consequence of coronary bypass surgery. Chronic failure means a person’s kidneys have essentially ceased functioning. And that means frequent dialysis: a time-consuming treatment that keeps fewer than 20 percent of patients over 65 alive for as long as five years. “The prognosis for a dialysis patient is frightening,” says Jeffrey T. Borenstein, director of the biomedical engineering center at the Charles Stark Draper Laboratory Inc., in Cambridge, Mass., who is developing a replacement kidney.
Developers of biohybrid kidneys are targeting acute kidney failure first, because those patients are hospitalized and can be treated with a device that functions outside the body. Later, the developers hope to use what they learn to devise implantable devices that might extend the lives of those with chronic kidney failure.
If our friend Bob developed acute kidney failure today, perhaps following a coronary bypass, the most advanced treatment he could receive might include an experimental renal assist device (RAD) developed by H. David Humes, a professor of internal medicine at the University of Michigan, in Ann Arbor. The RAD is now in its second of three phases of clinical trials for use as an adjunct in treating acute kidney failure.
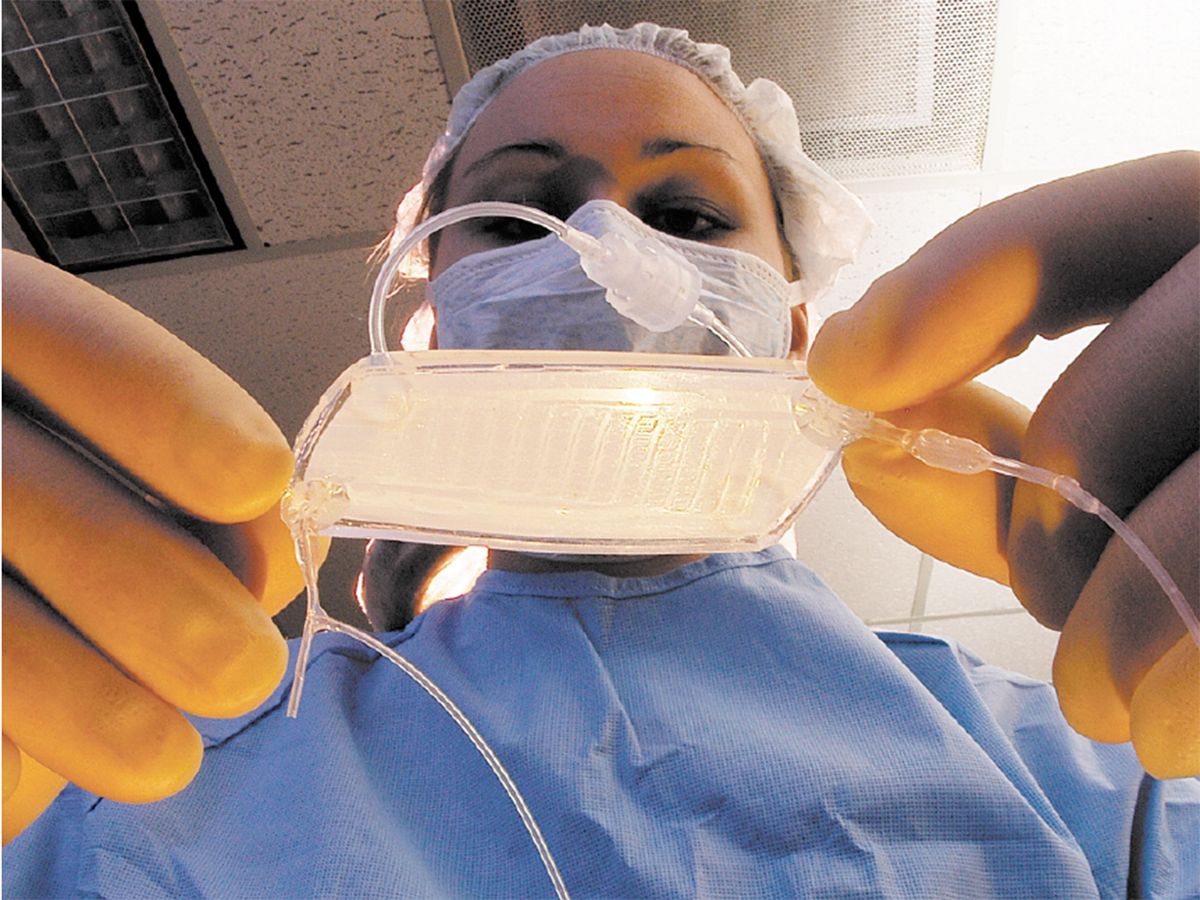
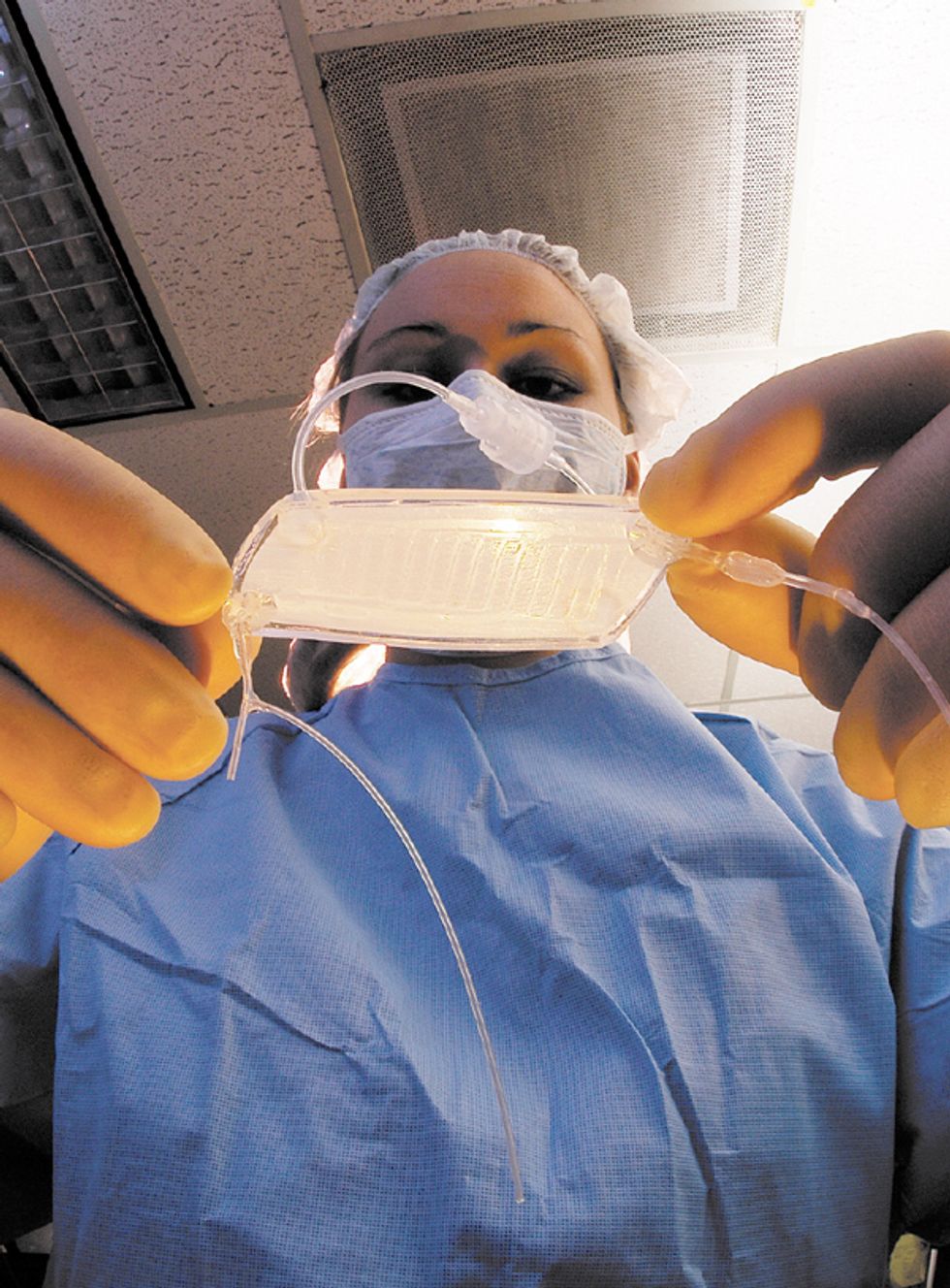
The RAD is a device that can be hooked up to a dialysis machine at a patient’s bedside [see illustration, "An Unconventional Kidney"]. It’s a biohybrid because it contains human proximal renal tubule cells, kidney cells that normally recover useful chemicals from the fluid that remains after the blood has been strained through the glomeruli, the kidney’s first line of filters. The glomeruli act conservatively but usually filter out some beneficial compounds along with the bad. To counteract that, cells in the renal proximal tubules scavenge glucose and salts, in particular, from the filtered fluid and return them to the blood. The cells also appear to help the immune system fight off systemic infections, a major cause of death from acute renal failure. The RAD, containing proximal renal tubule cells taken from kidneys that couldn’t be used for transplant, is designed to do the same. It filters fluid that has already gone through a dialysis machine, which also removes some useful substances.
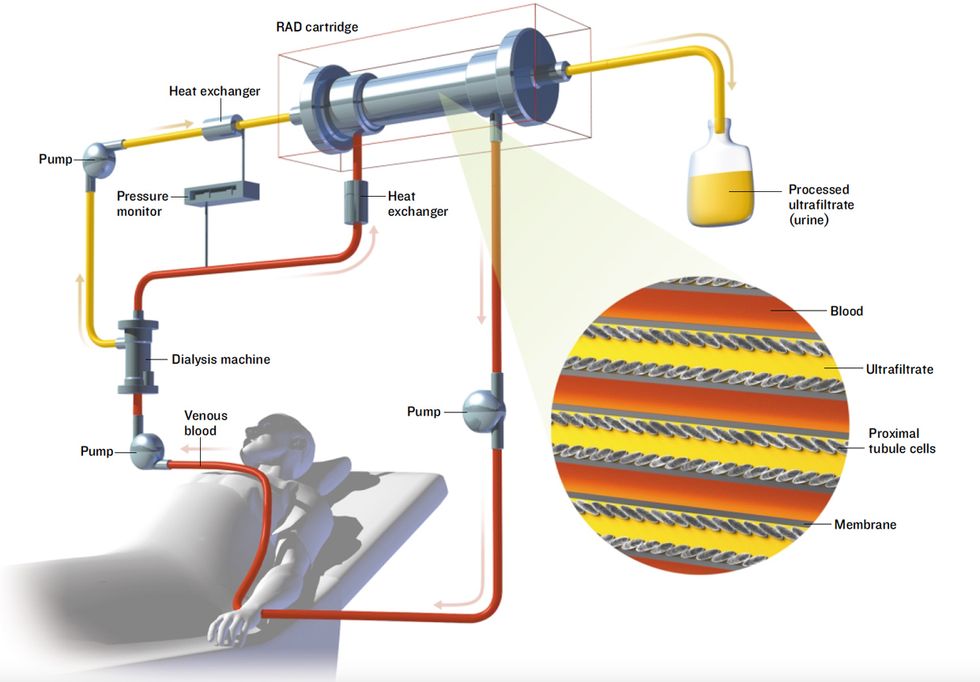
An Unconventional Kidney
The renal assist device (RAD), under development at Nephros Therapeutics, is a bioartificial organ being tested as an improvement on conventional kidney dialysis. In early trials, just a day or less of treatment with the RAD extended the life of critically ill patients by weeks.
Blood is pumped from a patient’s arm through a kidney dialysis machine, which filters waste material from the blood. Unfortunately, some useful substances are also filtered out. So the filtrate and blood are fed through the RAD. The RAD contains renal proximal tubule cells that take the useful substances from the filtrate and put them back into the blood, which is then pumped back into the patient.
In its Phase I clinical trial, the RAD was used in combination with kidney dialysis to treat 10 patients with acute kidney failure for up to 24 hours. All but one of the patients was expected to die within days; instead, six survived for weeks. While it was hooked to a patient, the device boosted kidney function and urine output and seemed to help the patient’s own kidneys recover, Humes says. He founded Nephros Therapeutics Inc., Lincoln, R.I., to develop the device.
Ultimately, Humes would like to transform the RAD into part of an implant for treating chronic renal failure. A nephrologist who collaborates with Humes, William H. Fissell, has begun to grow renal proximal tubule cells on silicon chips to create such a device. One of the toughest challenges will be making something small enough to be implanted in someone’s body but large enough to filter the roughly 180 liters of blood processed each day by a person’s two kidneys.
Borenstein, from the Draper Labs, is working on the scale-up problem. He recently reported initial results from his prototype kidney system, a microfluidic device that has an area of 15 square centimeters and is about 1 millimeter thick. The device mimics the structure of the glomeruli, which filter the blood before it passes through the renal proximal tubules in the kidney, and consists of two planar sheets of plastic etched with microchannels and separated by a special polymer-based filtration membrane. As blood courses through the microchannels in the top sheet, waste products pass through the membrane into the microchannels of the bottom sheet. “You scale up by adding layers,” says Borenstein, who began his career as a physicist in the semiconductor industry. The ultimate device might have hundreds or thousands of layers, he predicts. In the tests, he demonstrated that the MEMS could remove creatinine and urea from blood samples—two of the three major waste products filtered by the kidneys.
As a next step, Borenstein plans to incorporate renal proximal tubule cells into the MEMS. He foresees creating a device attached to the circulatory system—perhaps at the wrist—that would be driven by the body’s own blood pressure.
If Bob ever had viral hepatitis, he might eventually need a liver transplant, because the damage wreaked by hepatitis viruses often leads to liver failure years later. Most researchers agree that the liver’s complex digestive, hormonal, and metabolic functions will make it tough to replicate. But since the demand for liver transplants is so high—at roughly 17 500 in the United States, second only to the need for kidneys—several academic labs are trying anyway. They are pursuing bioartificial livers by building tiny channels in MEMS to mimic the liver’s microvasculature.
One notable laboratory in the search is led by Joseph P. Vacanti, a professor of surgery at Massachusetts General Hospital, in Boston. Vacanti’s group uses patterned silicon as a master mold to produce thin sheets made of biodegradable polymers such as polylactic glycolic acid (PLGA), the same material from which dissolvable sutures are made [see illustration, "Layers of Liver"]. “You pour [the PLGA] on the surface of the silicon, let it dry, and then peel it off,” Vacanti says. The end result is a micropatterned substrate with tiny channels that mimic the microvasculature feeding the liver or the tracery of bile ducts that lead to the digestive tract.
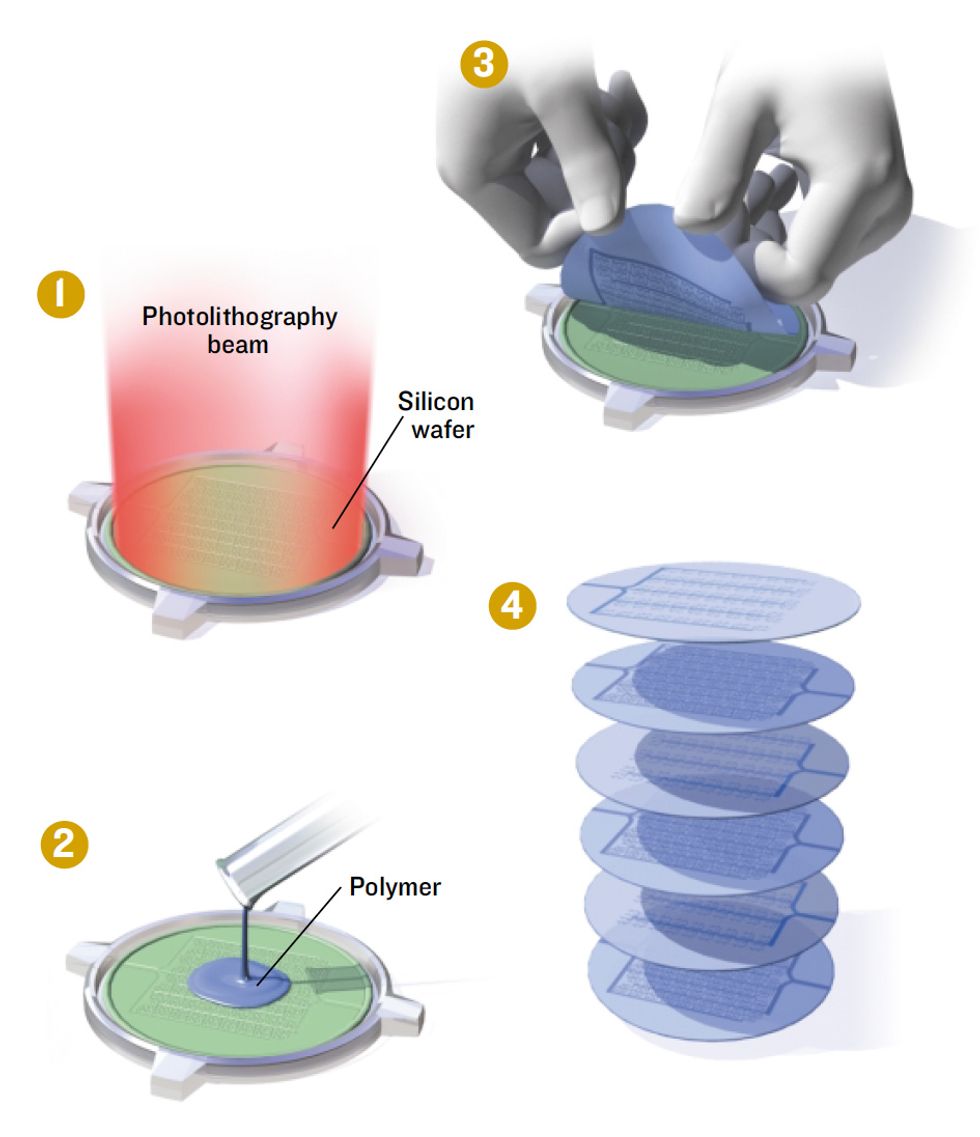
Layers of Liver
An experimental scheme constructs a bioartificial organ out of multiple layers of patterned biodegradable polymer.
1. Photolithography defines a pattern of microscopic blood vessels and other structures on a silicon wafer that will act as a mold.
2. A biodegradable polymer is spread over the mold and allowed to set.
3.The polymer is peeled off the silicon.
4. Polymer layers are seeded with the various types of liver cells needed, and then stacked and sealed to form a complete biohybrid liver.
Each liver module consists of two layers of PLGA. One is seeded with liver cells. The other is honeycombed with channels for blood flow that are lined with endothelial cells, the primary constituent of blood vessel walls. Separating the two is a thin polymer membrane through which proteins and toxic substances can pass to be broken down or detoxified. The support layers are biodegradable, because once the bioartificial liver matures, the hope is that it will be able to support itself without the PLGA.
The resulting biohybrid liver module overcomes an early stumbling block of tissue engineers: you need lots of cells in order to simulate an organ, but the more tightly you pack them, the farther they get from a blood supply, so they run out of oxygen and starve to death. In Vacanti’s devices, “the cells are never more than one membrane away from their blood supply,” he says.
Vacanti reports that his group is working to stack the liver modules in sufficient quantities that they could take over for an adult liver. So far, their top number is 10, however, and the job might require as much as 100 times that. The group is evaluating modules implanted in animals, and it plans to report its results within a few months. If the studies pan out, Vacanti says, the next step might be to test the modules as a means of keeping patients alive while they wait for traditional liver transplants. Eventually, he says, he hopes the devices might substitute for transplants. “We see this technology evolving into implantable biohybrid devices and, ultimately, into organs made entirely from tissue,” he says.
Although not implantable, a biohybrid liver-assist device has already made it out of the lab. But its producer, Circe Biomedical Inc., in Lexington, Mass., recently went out of business. Circe’s devices were composed of plastic pipes packed with straws made of an artificial membrane material and filled with a type of human liver cells, hepatocytes. Patients were hooked up to external pumps that ran their blood around the straws to be cleansed by the hepatocytes, after which the blood was returned to the body. Circe’s human trials showed, however, that the device worked only in the small subset of people with fulminant liver failure, a condition that kills hundreds of people each year and is characterized by brain swelling. When the U.S. Food and Drug Administration (FDA) demanded additional trials, the added expense knocked Circe out of business.
“A lot of things that seemed to work haven’t made it through the FDA, because small companies just don’t have the money or the skill sets to get them approved,” says Michael J. Lysaght, director of the Center for Biomedical Engineering at Brown University, in Providence, R.I.
Bob smoked for 30 years before finally quitting a decade ago, so he’s at risk for developing some type of chronic obstructive pulmonary disease (COPD), such as emphysema, which kills roughly 100 000 Americans each year. Along with severe infections, it is a primary disorder requiring lung transplants, and both conditions are more likely among the elderly. The demand for lung transplants tends to be a third of that for livers, but it will undoubtedly grow as the world population ages.
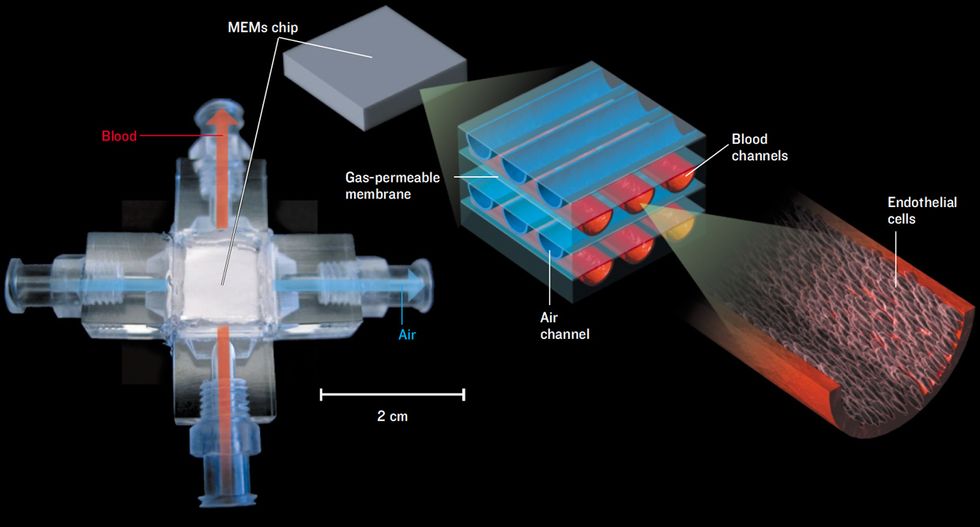
Bob might benefit from an implantable biohybrid lung being developed by associate professor William J. Federspiel and graduate student Kristie Henchir, both at the McGowan Institute for Regenerative Medicine at the University of Pittsburgh. The biohybrid attempts to emulate the gas-exchange function of the lungs, in which oxygen is absorbed into the blood and carbon dioxide is released. Lungs are so efficient at their job because inhaled air ends up in tiny sacs called alveoli, which are coated with a fine net of capillaries. Accordingly, blood and air are separated by only a thin membrane, through which gases can diffuse.
The biohybrid lung is based on a MEMS device, about the size of a thick credit card, that duplicates the function of the alveoli by bringing air and blood into close contact [see illustration, "Breathing Chip"]. The MEMS is laced with microchannels containing either air or blood, which are separated by thin membranes that mimic the alveolar wall. To prevent coagulation, the blood-containing microchannels are lined with endothelial cells, past which the blood has evolved to flow smoothly without clotting. Federspiel and his collaborators use endothelial cells from discarded umbilical cords, but they eventually plan to use cells taken from a patient’s own fat tissue.
“We’ve been able to grow the cells in the channels, and we’re now looking to see what level of blood flow we can use and still keep the endothelial cells attached to the microchannels,” Federspiel says. “Once we get these little units perfected, we could integrate them into larger-scale modules that would be either implanted or worn outside the body.”
The ultimate biohybrid-organ design problem might be the heart, because it must beat steadily and continuously throughout a person’s entire lifetime—about 100 000 beats each day. Fabricating a complete heart made entirely from human cells turned out to be more difficult than expected. Instead, tissue engineers are now looking for ways to replace pieces of the heart.
Robert S. Langer, a professor at the Massachusetts Institute of Technology, in Cambridge, was a pioneer in this area. He and MIT principal research scientist Gordana Vunjak-Novakovic plan to begin animal tests soon for cardiac tissue constructs that she calls “contractile patches.” A decade or so down the road, if Bob were to have a heart attack—the leading cause of death in industrialized countries—he might turn to Vunjak-Novakovic and her colleagues for a living bandage. The MIT group is working to make a patch of muscle that would be surgically attached to a damaged part of the heart to take over its contractile duties.
The MIT researchers have been able to coax heart cells isolated from newborn rats to mature into cardiac muscle by placing them on support scaffolds of biocompatible materials and exposing them to electrical stimulation and plenty of oxygen. Under these conditions, the heart cells fuse into functional tissue and begin beating synchronously. It works because the heart is essentially an electrical organ, crackling with current during each beat. “We trick the cells by using the same electrical stimulation they would get during development,” Vunjak-Novakovic says. The result is tissue that looks and acts just like slices from a mature heart. “When we compare the engineered tissue to native tissue, we see no essential differences,” she says.
To produce their contractile patches, she and her colleagues take a biodegradable polymer invented at MIT, called biorubber, and use a laser to pierce it with a fine network of channels [see illustration, "Mending a Broken Heart"]. Each piece is a rectangle roughly 1 cm2 in area and up to 3 mm thick. Next, they seed the biorubber with the three major cell types of a heart: cardiomyocytes, endothelial cells, and fibroblasts. (Human cells of these types can be derived from so-called adult stem cells, found, for example, in leftovers from liposuction.) As the cardiomyocytes beat, they adhere to and tug on one another, helping them to communicate electrically and to secrete the growth hormones they need to survive.
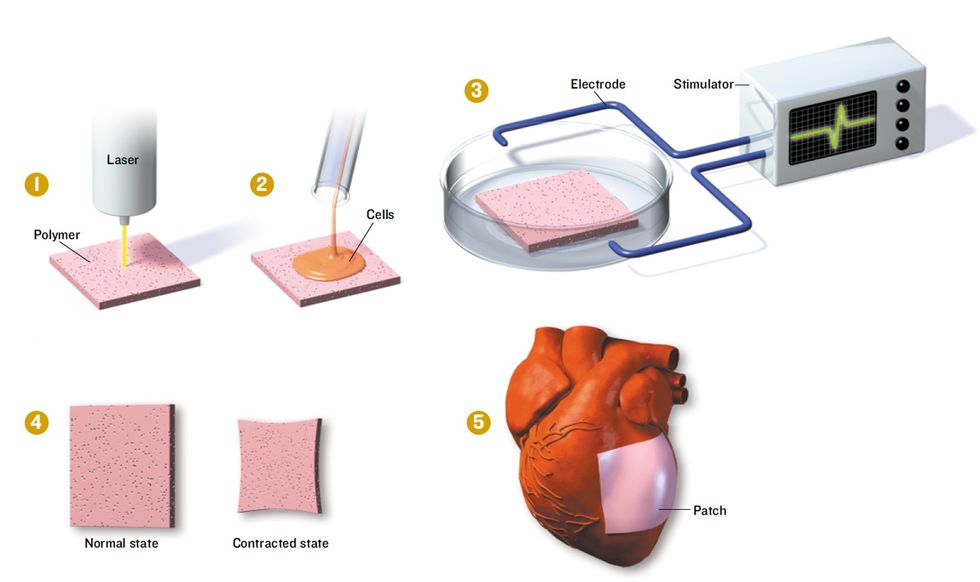
Mending a Broken Heart
Researchers at the Massachusetts Institute of Technology are trying to engineer patches of heart muscle to replace those areas damaged during heart attacks.
1. A thin square of a biodegradable polymer is shot through with a laser to form a fine network of channels through which fluid can flow.
2. The polymer patch is then seeded with the three major types of heart cells.
3. To simulate the conditions in a living heart and coax the cells to form functioning heart muscle, the patch is stimulated with electric current.
4. When mature, the patch beats like a piece of living heart.
5. Once a way is found to increase the thickness of the experimental patches, they will be strong enough to be grafted onto human hearts.
To be strong enough to replace dead heart tissue in people who have had heart attacks, however, the contractile patches must be at least 5 mm thick. Once they can achieve that thickness, Vunjak-Novakovic says, making patches of the needed size should be a cinch. “It’s relatively easy to grow a larger rectangle,” she says.
Of course, technical hurdles are only part of the biohybrid story. There are real concerns about how feasible biohybrid organ replacements will be as treatments for the disorders of aging. Right now, people older than 65 do not receive as large a proportion of organ transplants as one might expect, because policymakers have deemed that such scarce resources should go to younger, healthier people who have more years ahead of them.
Although that scenario could change if biohybrid organs ultimately become widely available, there are also the staggering costs to consider. Insurers are already reluctant to pay for organ transplants because they cost hundreds of thousands of dollars. Biohybrid organs could only be more costly because, unlike ordinary transplants, they must be manufactured.
With the intensive work being done on biohybrid organs, Bob—if he has the money and years more to live—might rest a bit easier knowing that replacement organs are on the horizon. But tissue engineers are wary of making bold predictions about how soon Bob and his friends might benefit from their technology. As Vunjak-Novakovic says, “We are at the end of the beginning, but there is a lot more to be done.”
About the Author
Carol Ezzell Webb is a freelance journalist in Austin, Texas, and a former editor at Scientific American. Her last article for IEEE Spectrum [“Chip Shots,” October 2004] described semiconductor-related drug delivery systems.
To Probe Further
Nephros Therapeutics (https://www.nephrostherapeutics.com/) is commercializing H. David Humes’s biohybrid kidney. Gordana Vunjak-Novakovic’s most recent cardiac muscle-patch research was published in December on the Web site of the Proceedings of the National Academies of Science (https://www.pnas.org).