Balancing Act
Noise is the key to restoring the body’s sense of equilibrium

Hold your arms outstretched, close your eyes, and then slowly and deliberately bring your index fingers together until they touch. Even though each fingertip is at the end of a long, multijointed appendage that operates freely in three dimensions, most people successfully touch their fingertips, with a positional error of 5 millimeters or less.
It’s a really simple thing for most of us to do. Yet it is a compelling demonstration of how sophisticated the body’s movement-control systems are. This natural motion control is made possible by an array of specialized sensory cells that are embedded in skin, muscles, ligaments, and tendons. These mechanoreceptors, as they are called by neuroscientists, convert mechanical phenomena—such as touch, pressure, muscle stretch, tendon force, and joint angles—into streams of nerve impulses that can be interpreted by the brain and spinal cord, which make up the central nervous system. Based on this rich flow of sensory data, muscles can be commanded to contract with the synchrony and precision to hit a curve ball or play the piano.
It is in many ways a classic feedback control system, similar to those that engineers design and use every day. But it is a controller largely hidden from us in the central nervous system, and we are rarely conscious of it. If you concentrate, however, you can definitely pick it up.
For example, try standing perfectly still. It’s impossible to remain truly motionless like a mannequin; your body naturally sways. If you pay attention to the pressure on the soles of your feet, for instance, you will become aware of an ever-shifting center of pressure. As your body leans forward, the focus of pressure travels out to the toes. Lean backward and the pressure moves toward the heels. Your sensory-motor control system integrates this touch sense from the soles of your feet with other balance feedback to keep you from falling over.
Given the critical role that the mechanical senses play, any loss of function involving them can obviously impact your health and quality of life. Most people expect that their vision and hearing will degrade with age but don’t fully grasp that the same holds true for their mechanical senses. For the elderly, the decline of the sense of touch in the feet and of proprioception—the sense of what position their limbs are in—is a strong contributor to the tendency to fall. In the United States, roughly one-third of people over age 65 fall each year, and many of these falls result in serious and debilitating injuries, such as broken hips.
Disease, too, can blunt the mechanical senses. Diabetes, for example, often leads to a generalized loss of nerve function, which in many cases manifests itself as a profound drop in mechanoreceptor performance. Lack of mechanical awareness, especially in the feet of people with diabetes, contributes to the occurrence of open sores that can be extremely difficult to heal and that all too often lead to the amputation of affected areas.
With literally millions of people around the world suffering from loss of sensitivity in mechanoreceptors, there is a huge opportunity for new technologies and therapies to improve the lives of these people and make a sizable dent in soaring health care costs. With those goals in mind, our research teams at Afferent Corp., in Providence, R.I., and Boston University have been collaborating to develop and test a new class of neurotherapy devices that have the promise of directly improving mechanical sensory function to help prevent falls in the elderly and foot injuries and amputations in people with diabetes.
These devices are based on the discovery, almost a decade ago, that certain forms of electrical or mechanical stimulation applied to mechanoreceptors increase their ability to detect sensory information. In effect, it is possible to turn up the volume on sensory signals from the extremities to increase input to the brain and improve sensory-motor control.
The starting point for understanding this means of sensory volume control is the fact that all sensory cells are so-called threshold-based units. That is, the stimulus from the environment must exceed a minimum threshold to cause the neuron to begin signaling with the rapid-fire voltage spikes it uses to communicate with the spinal cord and brain. One way to characterize the degradation of sensory function due to aging and disease is as an elevation in this threshold; stimulus levels that once were above the sensory threshold are now below it and cannot be felt.
The fundamental concept behind the new sensory enhancement technology is that it is possible to fill that subthreshold region with artificial activity, effectively providing a pedestal or bias of background activity to sensory neurons. This artificial stimulation does not itself cause the sensory neurons to fire—you can’t feel it, in other words. Rather, it puts the neurons in a state that predisposes them to fire when presented with a real stimulus from the environment—pressure on the sole of your foot, for instance. The result is that the neuron’s threshold of sensitivity is effectively pushed back down toward a normal, more sensitive level.
Interestingly, both mechanical and electrical forms of subthreshold stimulation improve sensitivity. This stems from the fact that each mechanoreceptor is a transducer that provides the interface between the mechanical environment we navigate through and our electrical-based nervous system. You can therefore push the neuron toward firing, either by presenting low-level mechanical energy in the form of slight vibrations or by inputting minute, submilliampere electrical currents.
But a counterintuitive finding has emerged from our research. The best type of stimulation signal is not a finely tuned frequency but rather noise—specifically, white noise, a signal comprising all frequencies within a certain band, in this case, typically less than 1 kilohertz. Given that engineers are trained to remove noise from systems to improve their performance, it may seem strange indeed that this neurological system seems to work best when noise is present.
It is not that sensory neurons defy traditional notions of signal-to-noise ratios. Instead, the dead zone below the threshold of sensation provides an opportunity for certain levels of noise to improve performance [see illustration, Feel the Noise (PDF)]. If the noise level is too high, the sensory neuron fires mainly in response to the applied noise instead of to the signal to be detected and, as with any sensor, the noise degrades its performance. But just the right amount of noise provides the pedestal upon which signals can ride over the threshold. The use of noise to improve the performance of nonlinear systems like this one is termed stochastic resonance.
Stochastic resonance gets its name from the stochastic, or random, signals involved and the fact that, as in a resonance phenomenon, you can get a bigger than expected impact from small-amplitude signals. Its origins lie about as far afield from both engineering and medicine as one can get. In the early 1980s, physicists at the Free University of Brussels and the University of Rome, La Sapienza, were trying to explain our planet’s more-or-less regular ice ages, which occur about every 100 000 years. The frequency of those episodes matches a periodic elongation in Earth’s orbit, but by itself the elongation is too small a factor to bury the world in glaciers.
The physicists figured that random climatic fluctuations—atmospheric noise—could combine with the periodic orbital force and push Earth’s climate into or out of an ice age. In the following years, scientists found instances of stochastic resonance in phenomena as diverse as chemical reactions and the behavior of lasers.
Stochastic resonance remained largely the purview of physical systems until 1993, when Frank E. Moss, a physicist at the University of Missouri, St. Louis, found that sensory neurons connected to the fine hairs on crayfish tails appeared to use stochastic resonance. Crayfish use the tail hairs to detect disturbances in the water that might indicate a predator’s presence. But these disturbances are so weak that you would expect roiling water in streams to hide the predator’s signal and leave the crustacean open to attack.
But crayfish sensory neurons, Moss discovered, actually take advantage of the noisy signal of a stream’s turbulence, using it to amplify the predator’s prowlings. A few years following Moss’s report, our lab at Boston University showed that stochastic resonance also works in the human nervous system. We showed that a person’s sense of touch was better when a little random vibration was applied to the fingertips.
But why noise? Why wouldn’t applying a constant light load or small dc electric current work? The answer lies in a basic feature of sensory neurons: they are excellent at adapting to constant or regular periodic input. When presented with such a stimulus over an extended period, the neuron adapts to the stimulus and ceases to respond. People become gradually unaware of, say, the touch of clothes on their skin. If the input signal is noisy and random, neurons are unable to adapt to it.
To prove that someone’s sense of touch could be improved, we had to first find that person’s sensory limits. Clinicians routinely do this by poking patients with either a standard kit of filaments of different stiffnesses or a vibrating probe. Finding the level at which the patient feels the force of the filament or the vibration of the probe quantifies that patient’s sensitivity. By recording a person’s performance while randomly switching the stimulation on and off, we can show whether the noise boosts sensitivity.
In experiments we performed in collaboration with Aristidis Veves at the Joslin-Beth Israel Deaconess Foot Center, in Boston, we showed that we could improve sensitivity on foot soles of people with diabetes through the use of vibrating insoles. The vibrations were so small that the patients could not feel them, removing any chance that they would be biased in favor of the noise.
These findings complement earlier work by our group showing that imperceptible random vibration could enhance sensitivity on the fingers of the young, the elderly, and stroke patients, as well as those with diabetes. Future research will focus on the ability of diabetic subjects wearing vibrating insoles to improve their gait and walking patterns to minimize uneven pressure points.
We’ve gone a step further, so to speak, from just measuring sensitivity in our work with older people. Here we are looking at how a little noise can help them stay on their feet. We hypothesized that a heightened sense of touch on the soles of the feet can be integrated into the sensory-motor control system that allows people to maintain their balance while standing still.
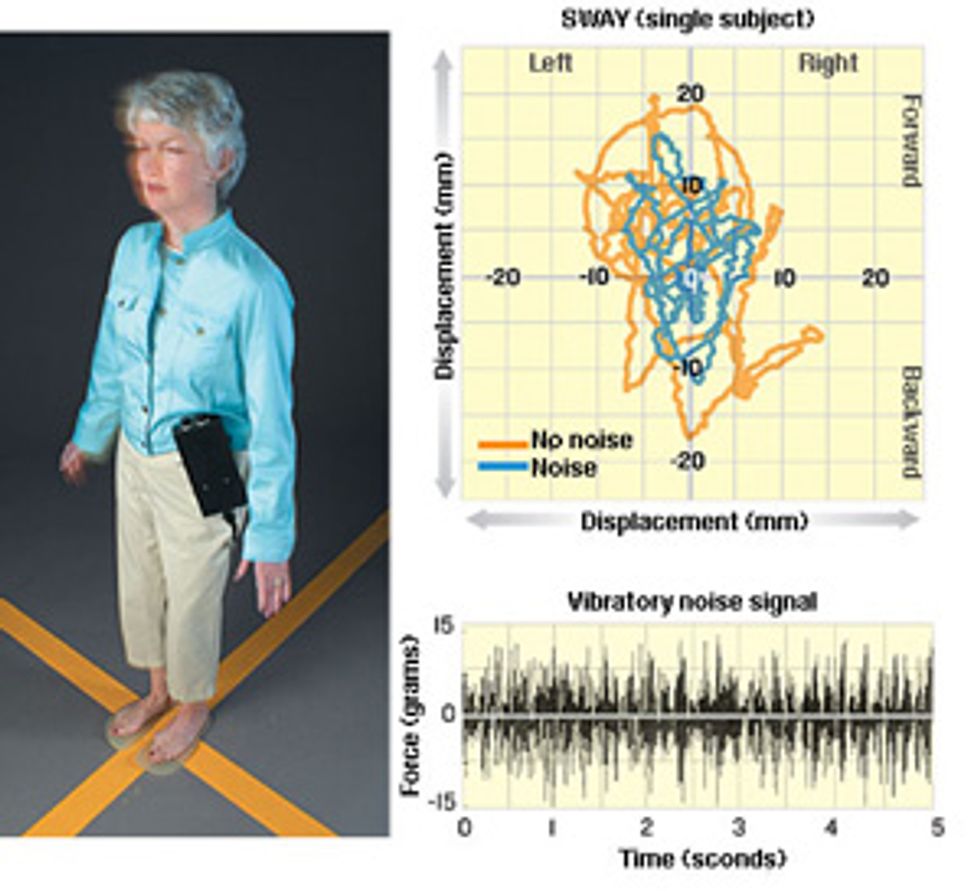
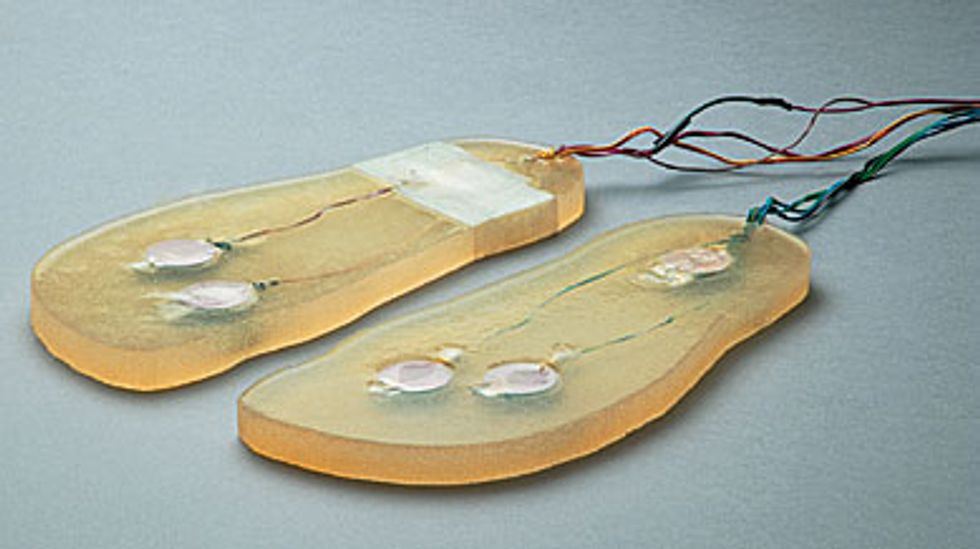
Still Standing: Using a phenomenon called stochastic resonance, the human body can make use of random vibrations to help maintain its balance. In experiments on people in their 20s and people in their 70s, actuators embedded in gel insoles [see photo, "Vibrating Soles"] generated noisy vibrations [above] with such a small amount of force that a person standing on the insoles could not feel them. A reflective marker [not shown] was fixed to the research subject's shoulder, and a video camera recorded its position.
People always sway a small amount even when they are trying to stand still [orange line, top]. And the amount of sway increases with age. But under the influence of a small amount of vibration, which improves the mechanical senses in the feet, both old and young sway much less [blue line]. Remarkably, noise made people in their 70s sway about as much as people in their 20s swayed without noise.
We asked test subjects to stand on a pair of vibrating insoles, with their eyes closed [see illustration, "Still Standing"]. We set the amplitude of the vibrational noise to a low level and then slowly increased it until the research subject could feel it. (This level is different for each person.) Then we dialed down the amount of noise until it was just under that level, which effectively blinds the subject as to whether the stimulation is on or off during the trials. We put a reflective marker on the subject’s shoulder and tracked its horizontal position with a special camera system. In an attempt to quantify the subject’s balance from his or her posture, we plotted the shoulder marker’s movements while the noise was turned on and when it was off.
The result, called a stabilogram, is an incomplete measure of how stable the subjects were when standing—there are too many joints between the shoulder and the feet to fully analyze posture from it. Nevertheless, the movement at the shoulder can tell us important things, such as how much a person sways before the internal control system makes a correction. In general, a tightening of the stabilogram indicates greater stability.
We performed the experiments with people in their 20s as well as people in their 70s, and we demonstrated a significant improvement in balance control in both groups when noisy vibration was applied to the soles of the feet. In particular, we found that both young and old subjects swayed considerably less when the vibrating insoles were turned on.
The improvement was more pronounced for the older group—so much so that when the septuagenarians wore the vibrating insoles, they swayed similarly to the twenty-somethings without vibrations.
Our results also fit well with a model of the body’s balance control system we developed in the early 1990s. To bolster the model, we performed an analysis of our stabilograms that relied on the data’s similarities to the random motions of microscopic particles. The analysis suggested that the body uses not one but two types of control systems while standing. If it drifts off center for less than a second or for a small distance, the nervous system appears to be using an open-loop control system—that is, one that does not use sensory feedback to keep it on target. But over longer time periods and greater distances, a closed-loop, feedback-driven system appears to kick in and help the body right itself.
In the vibrating sole experiments, we performed the same analysis, which showed that people’s balance control systems seemed to be more tightly controlled when under the influence of noise. That is, when the insoles were vibrating, they swayed a shorter distance before feedback control helped correct their posture. Since we know that the vibrations enhance the sense of touch on the foot, the point at which the body switches to feedback-based control appears to be influenced by the sensitivity of mechanical sensors in the feet.
To make use of this discovery in a clinical device, you need to know two things: which body parts to stimulate to get the desired effect and which procedure will work best. To improve balance in the elderly or prevent foot problems in people with diabetes, the bottom of the foot has proven a good target. A noise-delivery device could be shoes with built-in stimulators or removable insoles.
Although both electrical and mechanical noise enhance the sense of touch, reliably delivering electric current to the skin on feet has proven difficult. So we have concentrated on mechanical stimulation, such as embedding small linear electromagnetic motors in gel-based insoles. The prototype we’ve used in recent experiments has two motors at the ball of the foot and one at the heel [see photo, "Vibrating Soles"]. The motors are externally controlled and powered.
But we must overcome several design challenges to go from this prototype to a commercial product. The device, ideally, will be a thin insole—consisting of motors, control electronics, and batteries—that can be inserted into most shoes. To that end, we are trying out a number of combinations of low-profile actuators with several types of gel. We are also looking into very thin batteries, but these may not have the energy density to deliver hours of vibration, so we might have to turn to external batteries.
It may be necessary to build a shoe instead of an insole, because there will be more room for the system, but then effort must be spent on considerations of fashion. In either case, comfort will be an important consideration. Because this technology is palliative—that is, it alleviates the symptom but doesn’t cure the cause—people will most likely need to wear these devices a good portion of the day, perhaps 8 hours or more. So we will have to be careful that the motors are placed in such a way that they do not themselves become irritating. Whatever the final configuration, we hope to have it approved by the U.S. Food and Drug Administration and on the market within two years.
You have to learn to crawl before you can walk, as the saying goes. For our work, we learned how to improve standing, so that we could go on to more challenging and clinically relevant problems, such as walking and climbing stairs. We’re also going to see if a little noise will help people recover from incidents such as tripping or being pushed. Some additional studies examining the effects of this stimulation on the balance of people with diabetes or those coping with the aftereffects of a stroke are under way, too.
Noise-based sensory enhancement technology was not originally intended to reverse or cure any underlying neurological dysfunction—but rather to help people live more safely and independently. However, there is a growing body of research that establishes the connection between sensory activity and the ability of the nervous system to repair itself following an injury.
The paralyzed actor Christopher Reeve, who died last year, became an example of this restoration, as his activity-based therapy was credited with the unprecedented return of his ability to feel a pin prick over most of his body. A new class of devices, now in limited testing, may be able to use the sensory-boosting attributes of our technology to help people relearn simple movements following a stroke and speed their recovery.
What’s more, stochastic resonance technology may not be limited to those with medical problems. Perfectly healthy people might someday benefit, too. Just as in recovering from a stroke, there is a chance that learning a new activity, such as an effective golf swing, may be accelerated by the heightened sensory awareness that this technology might provide. Indeed, almost anywhere you need sensory input in order to interact with the environment, a little noise might make things better.
About the Authors
James J. Collins is codirector of the Center for BioDynamics at Boston University. In 2003, he received a MacArthur Fellowship (a “genius” award). Jason D. Harry is CEO of Afferent Corp., a start-up medical device company in Providence, R.I. James B. Niemi is the director of engineering at Afferent. Attila A. Priplata is a graduate student in biomedical engineering at Boston University under Collins’s mentorship.
To Probe Further
Our results in the elderly were reported in “Vibrating Insoles and Balance Control in Elderly People,” by A. Priplata et al., The Lancet, 4 October 2003, pp. 1123-24.
For more on our experiments, see “Noise-Enhanced Human Sensorimotor Function,” by J. Collins et al., IEEE Engineering in Medicine and Biology Magazine, March-April 2003, pp. 76-83.
All of our research is listed at https://www.bu.edu/abl.
For information on stochastic resonance, see “Stochastic Resonance and Sensory Information Processing: A Tutorial and Review of Applications,” by F. Moss et al., Clinical Neurophysiology, February 2004, pp. 267-81.