One roadblock to shrinking present-day electronics is the relatively large size of their capacitors. Now scientists have developed new “superlattices” that might help build capacitors as small as one-hundredth the size of conventional ones.
Whereas batteries store energy in chemical form, capacitors store energy in an electric field. Batteries typically possess greater energy densities than capacitors—they can store more energy for their weight. However, capacitors usually have greater power densities than batteries—they charge and discharge more quickly. This makes capacitors useful for applications involving pulses of power.
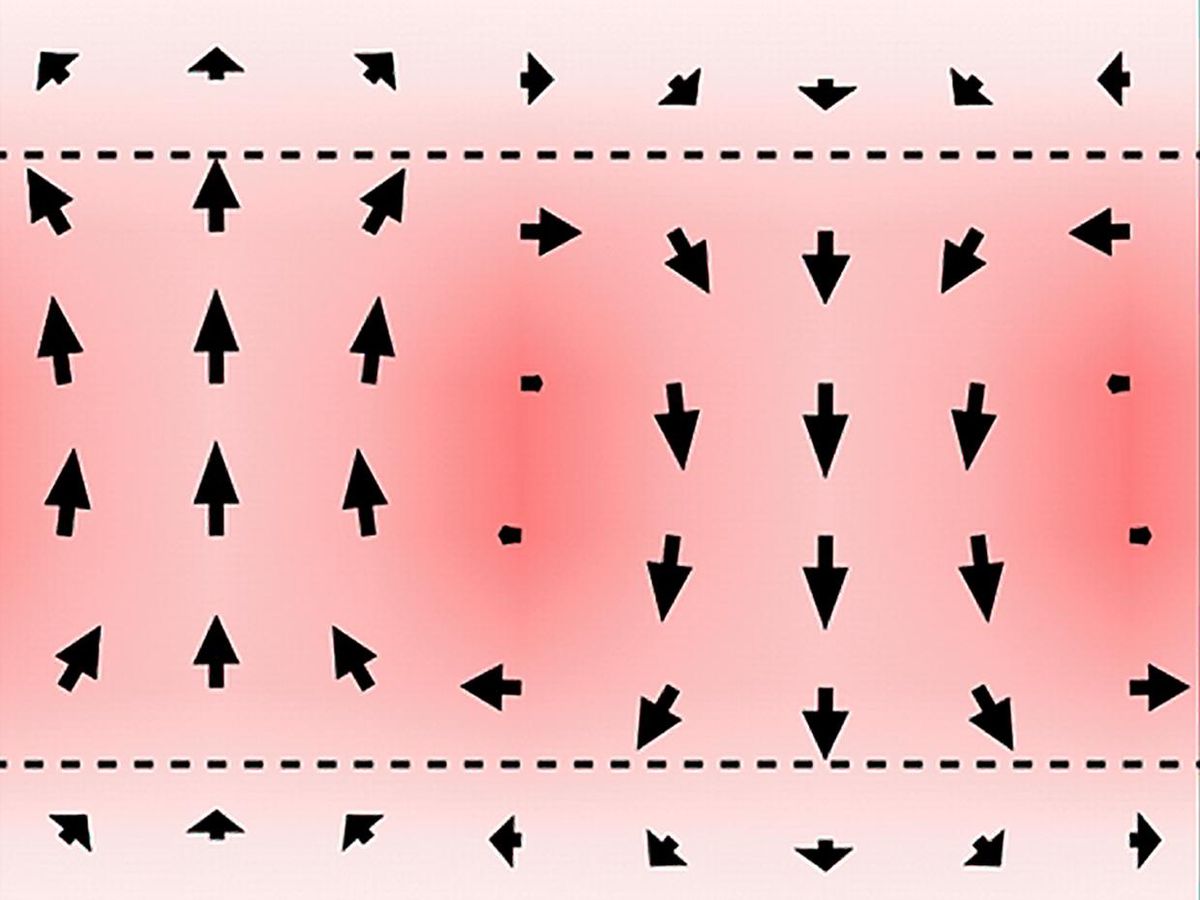
Since the energy density of capacitors is somewhat low, they are difficult to miniaturize. Antiferroelectric materials could help solve this problem. Like magnets, which possess north and south poles, electric charges within materials become separated into positive and negative poles. Antiferroelectrics are materials in which these “electric dipoles” are generally oriented in opposing directions, leading to an overall zero electric polarization. However, when antiferroelectrics are exposed to a strong enough electric field, they can switch to a highly polarized state, resulting in large energy densities.
“Capacitors made out of antiferroelectrics could be much smaller than conventional ones, which would help to miniaturize electronic circuitry,” says Hugo Aramberri, a materials physicist at the Luxembourg Institute of Science and Technology.
However, relatively few natural antiferroelectric materials are known. In a new study, Aramberri and his colleagues sought to engineer artificial structures that could act like antiferroelectrics.
The scientists first looked at ferroelectric and paraelectric materials. Ferroelectrics are materials in which the electric dipoles are all normally oriented in the same direction, resulting in electric polarization. In contrast, paraelectrics are materials in which the electric dipoles align only in the presence of an electric field.
The team then constructed superlattices made of ferroelectric lead titanate (PbTiO3) and paraelectric strontium titanate (SrTiO3). These superlattices got their name because the lead titanate and strontium titanate, themselves arrayed in lattice structures, are also placed in thin, alternating layers with each other.
The scientists sought to optimize their energy storage densities and energy release efficiencies at room temperature by experimenting with different features of the materials, including layer thicknesses, layer stiffnesses, and the strain that layers might feel from the foundations on which they rested.
In simulations, the scientists found that their best superlattice could store more than 110 joules per cubic centimeter, given an electric field of 3.5 megavolts per centimeter. This was better than nearly all known antiferroelectric capacitors at that field strength. It was surpassed only by a complex perovskite solid solution that displayed 154 J/cm3 at 3.5 MV/cm, currently the record-highest energy density at that field strength.
“Conventional capacitors have energy-storing densities that are more than 100 times smaller than the ones we predict for some of the artificial antiferroelectrics in the study,” Aramberri says. “This means that our superlattices could potentially be used to create capacitors with volumes 100 times smaller than the conventional ones.”
Aramberri notes there are factors other than energy density that one must consider when choosing a capacitor, such as its power density. “These deserve further investigation to assess the viability of our superlattices for commercial use,” he says.
He also cautions that the lattices they examine contain lead, whose poisonous nature strongly limits its technological applications.
“Still, we believe our work provides a proof of concept that artificial antiferroelectrics can be tailor-made out of ferroelectrics and paraelectrics, and this idea is not intrinsically tied to the particular materials chosen for the building blocks,” Aramberri says.
All in all, these new findings suggest that “for many practical purposes, we may be able to use tailor-made artificial antiferroelectrics instead of intrinsic antiferroelectrics,” Aramberri says.
The next step “would be to test our simulations in real samples,” Aramberri says. “It would be interesting to measure other properties, like how much voltage they can withstand, their endurance in long-term use, and ultimately commercial viability. The latter relies partly on scalable and inexpensive fabrication techniques of high-quality oxide superlattices, which are not fully developed yet.”
The scientists detailed their findings 3 August in the journal Science Advances.
Charles Q. Choi is a science reporter who contributes regularly to IEEE Spectrum. He has written for Scientific American, The New York Times, Wired, and Science, among others.