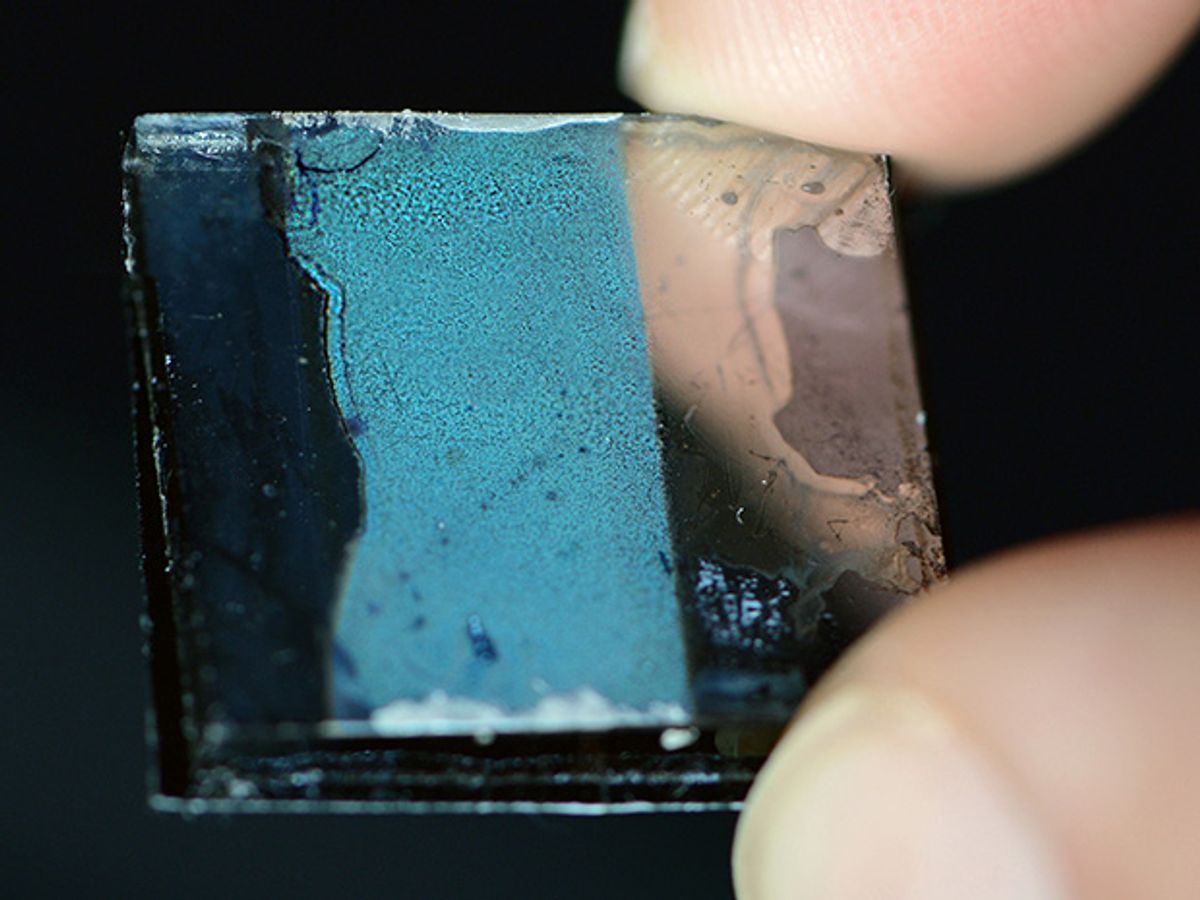
Researchers at the Technische Universität München (TUM) and the Ludwig Maximilian University of Munich have developed a new type of porous germanium with 200-nanometer pores giving it a large active surface area compared to its volume. This property is important for the exchange of electrical charges via a conducting surface in applications such as photovoltaic cells and batteries. The researchers, led by Thomas Fässler, a chemist researching novel materials at TUM, published their research in Angewandte Chemie Online earlier this month.
Currently, scientists use etching techniques to create nanostructured surfaces in germanium or silicon—a top down approach. The Munich researchers however used a bottom-up method based on the use of so-called Zintl clusters—charged structures of identical atoms discovered by the German chemist Eduard Zintl in the 1930s.
The germanium Zintl clusters, consisting of nine germanium atoms, were dissolved in a solution, and because they are charged, they repelled each other. Next, the researchers poured the solution over a template comprising a layer of closely packed polymer beads, 50 to 200 nm in diameter. The solution filled the spaces between the beads; when the solution was subsequently evaporated by heating, the germanium atoms merged and formed pure germanium—but studded with the beads. By dissolving the polymer beads, a pure germanium, spongy and lightweight, was left—with pores where the polymer beads had been.
Fässler notes that this is the first time such a porous material that is entirely inorganic has been created in this fashion. Further, he says, this porous semiconductor matrix can be doped by conventional means or by introducing dopants in the porous network, making it easy to tune its electrical properties.
Highly porous materials have been studied for their capability to transport charge carriers more efficiently in photovoltaic cells and batteries. Says Fässler:
Our overall goal is the creation of a hybrid solar cell; this means, the combination of an organic conductive polymer and a classical material—in our case, germanium. If you fill the pores with a conductive polymer, you can produce charge separation with sunlight, where the polymer produces a hole and the germanium uptakes an electron.
There are still hurdles. “The specific size of the pores is now 200 nm,” says Fässler. “In the long term we want to make those pores smaller. We built a first prototype, but the performance is low." He adds that he expects it to take a year to get a prototype with a new material to work.
Still, the German team’s research is on a fast track with a lithium-ion battery featuring a porous semiconductor electrode. Fässler explains that while other groups have used nanosize silicon or germanium particles, his group uses the inverse approach: producing nanoporous germanium instead of nanoparticles.
Currently, for the prototype, we use a germanium half cell on one side, and a pure lithium cell on the other side. This is our benchmark. The lithium will react with the walls of the pores and we have a much higher surface exposed to the lithium. Generally, if you use silicon or germanium in an anode, you need additional carbon with a high surface area to get a better conductivity. In our case, we can make a half cell without using any carbon.
Fässler reports that he and his collaborators are now doing cycling with this battery.
Silicon is the next major step in their research, but there are tradeoffs. “Actually, germanium is a better conductor,” says Fässler, “but silicon is much cheaper.” And although Zintl clusters for silicon exist, they are more difficult to make and to manipulate.