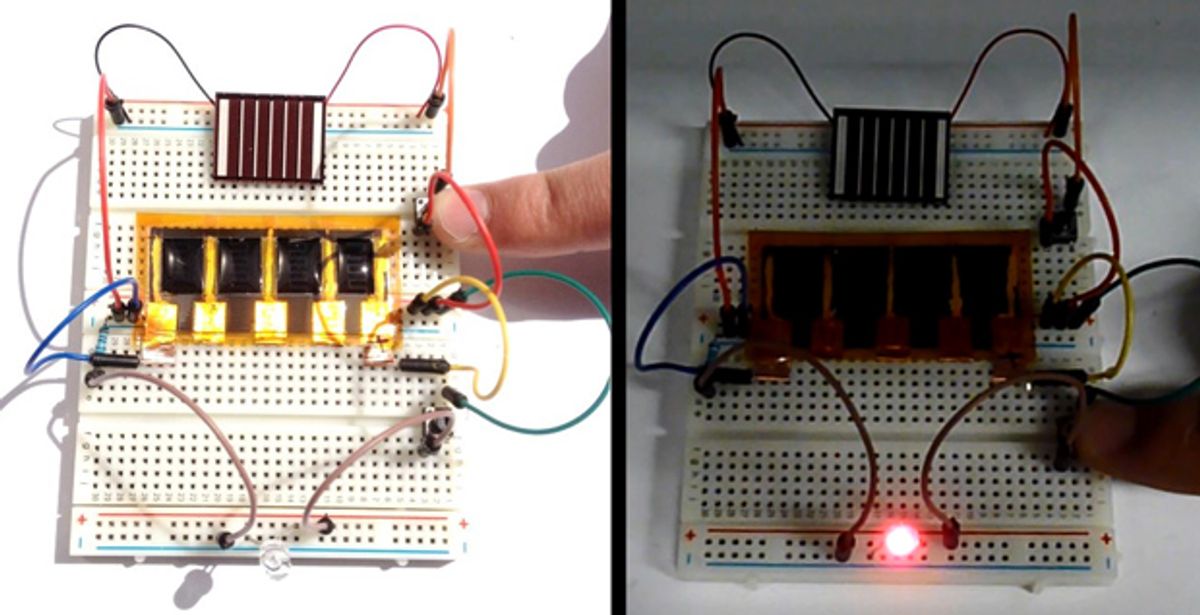
By combining sheets of graphene with a traditional battery material, scientists have created hybrid supercapacitors that can store as much charge as lead acid batteries but can be recharged in seconds compared with hours for conventional batteries.
Supercapacitors now play an important role in hybrid and electric vehicles, consumer electronics, and military and space applications. However, they are often limited in terms of how much energy they can store.
Now researchers at the University of California, Los Angeles, have developed a hybrid supercapacitor that is based on graphene, which is made of single layers of carbon atoms. Graphene is flexible, transparent, strong and electrically and thermally conductive, qualities that have led to research worldwide into whether the material could find use in advanced circuitry and other devices.
The scientists combined graphene with manganese dioxide, which is widely used in alkaline batteries and is both abundant and environmentally friendly. The manganese dioxide formed microscopic flowers made of flakes only 10 to 20 nanometers thick. The supercapacitors also incorporated electrolytes that can operate at high voltages.
The graphene provides a highly conductive structure for the manganese dioxide that is also very porous, helping ensure that more of the manganese dioxide can undergo electrochemical reactions. The resulting 3-D hybrid supercapacitor has an energy density of up to 42 watt-hours per liter, superior to many commercially available supercapacitors and comparable to lead acid batteries, the researchers said. Furthermore, the new supercapacitors can provide power densities up to roughly 10 kilowatters per liter, which is 100 times faster than high-power lead acid batteries and 1,000 times faster than a lithium thin-film battery. Moreover, the new devices could also retain their energy capacity over a cycle of 10,000 discharges and recharges.
The scientists demonstrated they could integrate their supercapacitor with solar cells for efficient solar energy harvesting and storage. They also noted their supercapacitors can be assembled in air without the need for the expensive dry rooms needed for manufacturing today's supercapacitors. They detailed their findings online March 23 in the journal Proceedings of the National Academy of Sciences.
Charles Q. Choi is a science reporter who contributes regularly to IEEE Spectrum. He has written for Scientific American, The New York Times, Wired, and Science, among others.