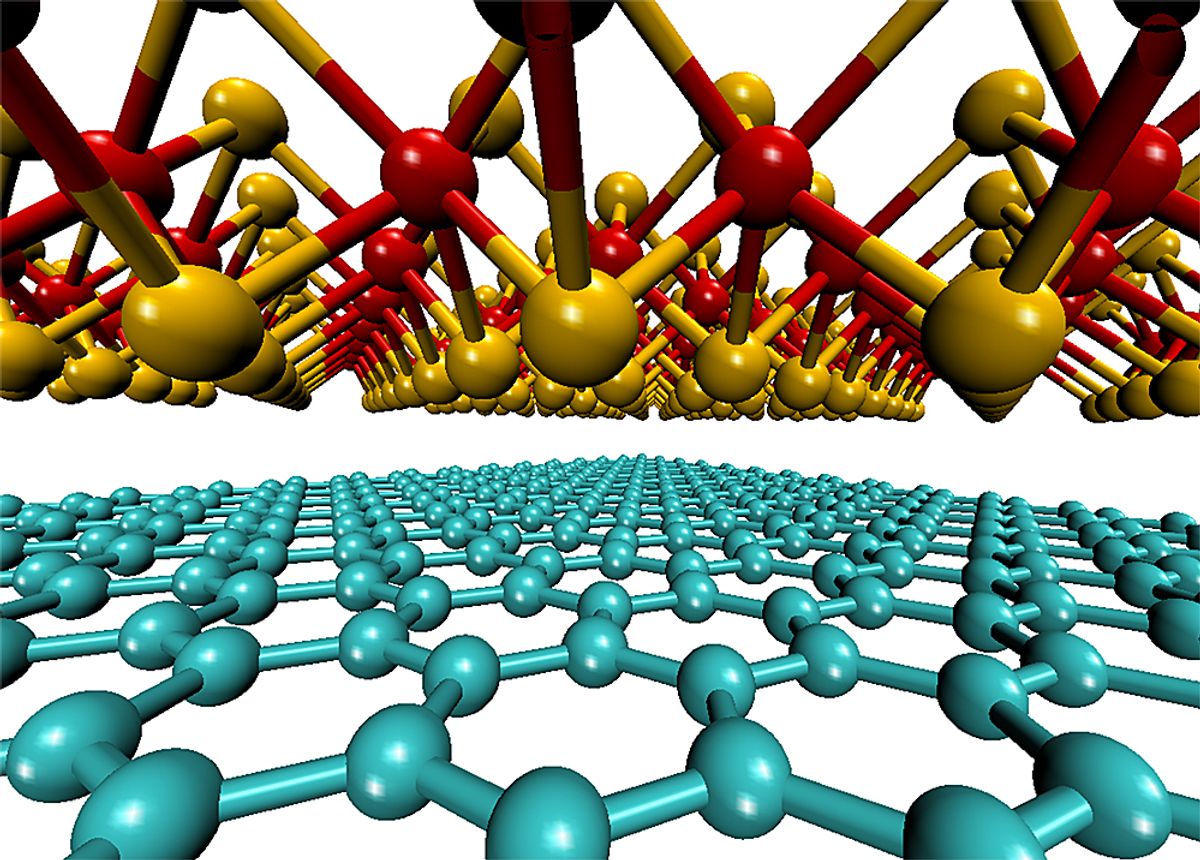
Soon after graphene was discovered back in 2004, a number of other two-dimensional (2-D) materials appeared, vying for the same attention. By now, the list has grown to a veritable catalogue of 2-D materials.
The story of these 2-D materials will ultimately be about how well they can adapt to different applications that we consider for them today, or may discover in the future. Of them, perhaps no area has been as hotly pursued as photovoltaics (PVs).
One interesting new potential application of graphene is in creating what is known as “hot carrier” cells in which the graphene produces, after absorbing one photon, is capable of generating multiple electrons instead of just a single one. But the main focus of applying graphene to PVs has been as a replacement for indium tin oxide (ITO) used in the electrodes of organic solar cells.
Now researchers at MIT are looking at 2-D materials, such as molybdenum disulfide and molybdenum diselenide, to make the thinnest and lightest PVs ever made. While this may not produce the highest energy conversion efficiency or be the cheapest material for PVs—the two typical metrics most sought after—they do expect that their lightness should create some possibilities in this application.
Graphene's own energy conversion capabilities are not what you would call impressive, and more generally, two-dimensional materials don’t really compete with the 18-19 percent conversion efficiencies of standard silicon cells already on the market. But in the research, which was published in the ACS journal Nano Letters (“Extraordinary Sunlight Absorption and 1 nm-Thick Photovoltaics using Two-Dimensional Monolayer Materials”), the MIT team demonstrated that if you stacked just three sheets on top of each other, a 1nm-thick stack can absorb up to 10 percent of incident sunlight, which is one order of magnitude higher than gallium arsenide and silicon. While the actual conversion efficiency is still pretty low at 1 percent, it does correspond to power densities being 100 to 1000 times higher than the best existing ultrathin solar cells.
“Stacking a few layers could allow for higher efficiency, one that competes with other well-established solar cell technologies,” said Marco Bernardi, a postdoc in MIT’s Department of Materials Science, in a press release.
Jeffrey Grossman, Associate Professor of Power Engineering at MIT and the paper's senior author, added in the release: “It’s 20 to 50 times thinner than the thinnest solar cell that can be made today. You couldn’t make a solar cell any thinner.”
Whether thinner and lightness will really translate into something that the market demands remains to be seen. The problem, as the researchers seem to concede, is that at this point there is no way to produce molybdenum disulfide and molybdenum diselenide in bulk. Until manufacturing techniques are developed that make that possible—and economical—the thinnest solar cells will likely remain laboratory curiosities.
Illustration: Jeffrey Grossman and Marco Bernard/MIT News
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.