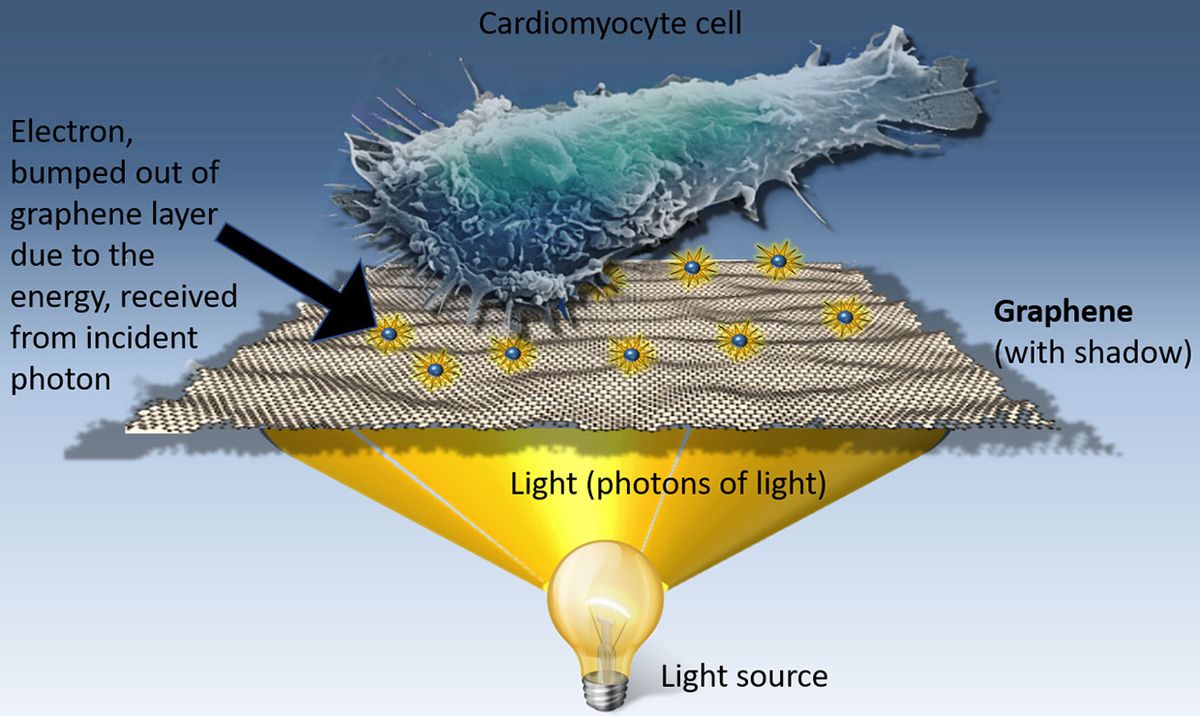
Shine light on human heart cells cultured on graphene and they beat faster. Shine light on zebrafish embryos with graphene flakes injected in their hearts, and the contraction of that organ speeds up.
That’s what scientists at the University of California San Diego reported today in the journal Science Advances, in a discovery they say has implications for everything from drug testing to pacemakers.
“Sometimes discoveries happen due to serendipity,” says Alex Savchenko, a biophysics researcher at the university, who led the discovery with Elena Molokanova at the San Diego-based startup Nanotools Bioscience. “In this case we were controlling what we wanted to achieve all the way through the experiment.”
Graphene, the wonder material composed of single atom-thick sheets of carbon, has been a focus of excitement and feverish development ever since some of its properties were first demonstrated, in 2004, by Andre Geim and Konstantin Novoselov, both now at the University of Manchester.
One of graphene’s many talents is that it can convert light into electricity. Savchenko and his colleagues hypothesized that the electricity generated by graphene could also stimulate human cells.
After honing the graphene formulation and trying out different types of light, Savchenko’s team managed to do what they set out to do: They built a gentle remote control for cell growth. Call it an opto-graphene stimulator.

“I was looking at the microscope’s computer screen and I’m turning the knob for light intensity and I see the cells start beating faster,” says Savchenko. “I showed that to our grad students and they were yelling and jumping and asking if they could turn the knob. We had never seen this possibility of controlling cell contraction.”
The discovery has these scientists dreaming of applying the device to a wide range of tools and therapies: Better stem cells, better drug screening, light-controlled pacemakers, devices that selectively destroy cancer cells based on their electrical potential, and pain killers that only work on people who are actually in pain.
That's quite a list—one that echoes the excitement over graphene generally. But all of these applications stem from the idea that culturing cells in a regular old plastic or glass Petri dish doesn’t mimic their natural environment very well—a challenge around which many groups are trying to innovate.
The heart, for example, runs on electricity. Every time it beats, a jolt of self-generated electricity runs through it. So if a researcher is testing a drug on heart cells, those cells should be getting jolts of electricity, just like they do in the body, says Savchenko. Testing a drug on those kinds of cells would give a more accurate indication of how a drug might actually behave in vivo, he says.
Or take pain for example. Scientists could emulate pain in the lab by stimulating neural cells in a particular way. That could allow them to test and develop an anti-pain drug that would respond specifically, or perhaps only, to cells in that condition. Such a drug would work only on people in pain, and not on people taking it recreationally—a smarter version of an opioid.
Another possibility is to use the graphene-based tool to selectively destroy cancer cells, while leaving nearby healthy cells unharmed. The electrical resting potential of cancer cells in many cases is lower than healthy cells. So it takes less stimulation to get the voltage-gated ion channels of the cell’s membrane to open up and let in positively charged ions. Graphene bits could be sent to a cancer site and turned on with light, delivering stimulation at a level that only affects nearby cancer cells. If their ion channels are held open long enough, the huge increase of ions in the cancerous cell will kill it.
Then there’s the potential for a graphene-based pacemaker that runs on light. Typical pacemakers are composed of gold and titanium, and after just a few years in the body, scar tissue can grow over the device and reduce the conductivity between it and heart tissue. A graphene pacemaker would be more biocompatible, reducing replacement surgeries, Savchenko says, and could run on an implanted light.
Savchenko’s team says their technique has an advantage over one of biotech’s hottest tools: optogenetics. In that technique, light is used to turn neural cells on or off. The catch: the cells have to be genetically engineered first to make them light-responsive. So the cells must be changed first before the investigator can control their behavior, and that might confound study results, the authors wrote in their paper.
Opto-graphene stimulation also has one up on traditional electrical stimulation. That decades-old technique delivers electrical pulses via electrodes, but can cause cell damage or death from high voltage pulses, Savchenko says. “It’s not natural. You’re burning the cells and they’re not feeling well afterward,” he says. “With graphene and light you can control extremely carefully the amount of electricity delivered.”
How exactly graphene converts light into electricity is still unclear. Savchenko has a theory, which he plans to present in an upcoming paper.
Editor’s note: This story was updated on 19 May to correct details about the release of Savchenko’s next paper.
Emily Waltz is a features editor at Spectrum covering power and energy. Prior to joining the staff in January 2024, Emily spent 18 years as a freelance journalist covering biotechnology, primarily for the Nature research journals and Spectrum. Her work has also appeared in Scientific American, Discover, Outside, and the New York Times. Emily has a master's degree from Columbia University Graduate School of Journalism and an undergraduate degree from Vanderbilt University. With every word she writes, Emily strives to say something true and useful. She posts on Twitter/X @EmWaltz and her portfolio can be found on her website.