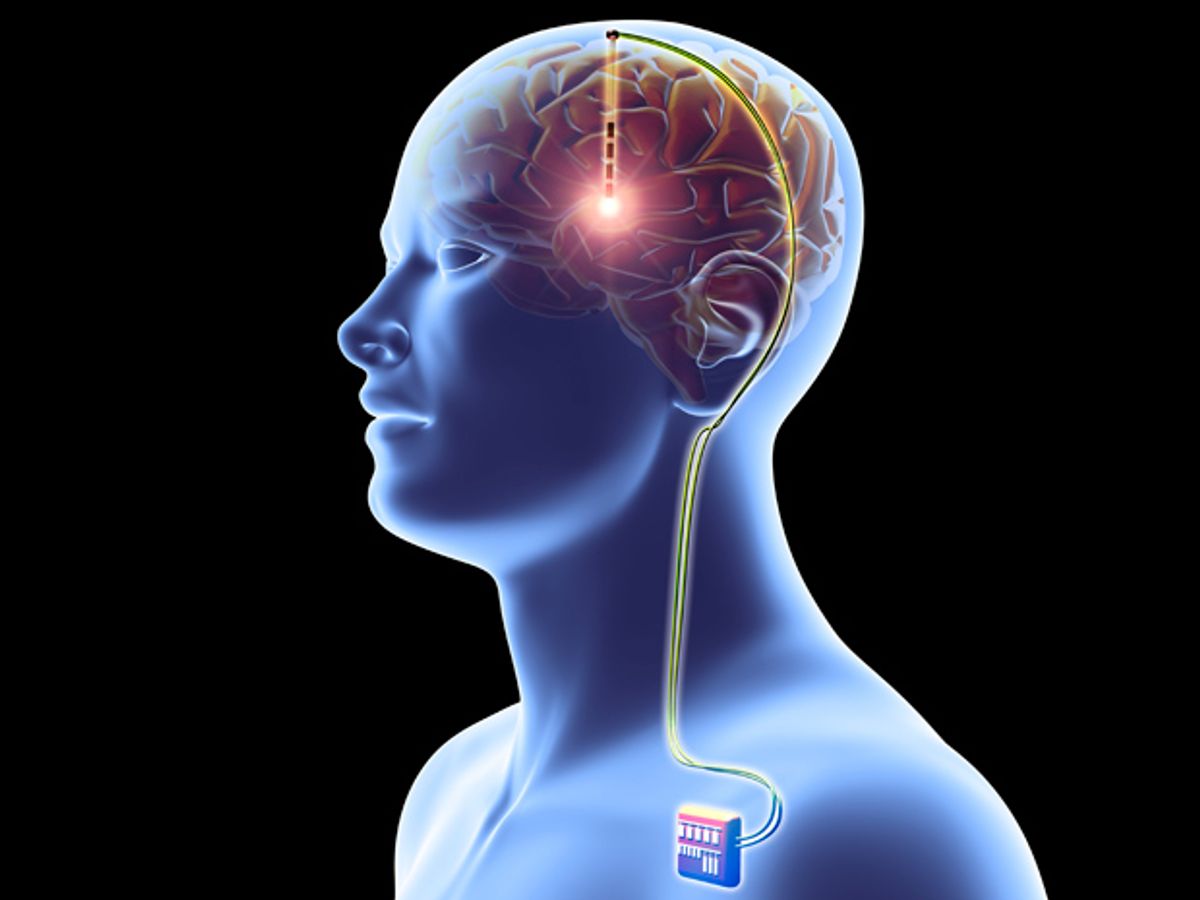
Doctors know that deep brain stimulation works as a therapy for Parkinson’s disease. But they’re still trying to figure out why and how. A new study sheds some light on the mechanism of action, suggesting that DBS disrupts a pattern of excessively synchronized activity in the brain.
In DBS, an implanted device sends tiny jolts of electricity through neurons, acting somewhat like a brain pacemaker. The technique is widely accepted as a treatment for Parkinson’s and other movement disorders; more than 100,000 patients have received implants that help control their tremors, rigidity, and other kinetic symptoms.
In recent years, researchers have also used DBS as an experimental treatment for psychiatric disorders such as depression, but the results have been mixed. Scientists are struggling to understand why some studies show remarkable results while others fail. Last year, DBS pioneer Helen Mayberg of Emory University told IEEE Spectrum that “the limiting factor is actually the neuroscience, not the engineering.”
The new work, led by Coralie de Hemptinne at the University of California, San Francisco, provided insight into the neuroscience by examining the brain activity of Parkinson’s patients on the operating table, while they were having their DBS systems implanted deep in the neural tissue. During a portion of the surgery in which the patients were awake, surgeons placed a strip of electrodes on the surface of each patient’s brain to record activity in the motor cortex. Then, by turning the DBS system on and off, the researchers could study the stimulation’s effect on neural activity there.
The electrodes record that activity in the form of brainwaves—or to put it more technically, oscillations of regular frequencies that represent millions of neurons acting in concert. De Hemptinne and colleagues had previously discovered that people with Parkinson’s disease have an abnormal amount of synchronicity [pdf] between low-frequency beta waves and high-frequency gamma waves. Specifically, the beta waves’ phase correlated with the gamma waves’ amplitude. In this new study, the researchers showed that DBS reduces this synchronization.
De Hemptinne explains that this phase-amplitude coupling, or PAC, is a normal feature of a healthy brain; it’s the excessive degree of synchronicity that poses a problem in the Parkinson’s brain. “In a healthy brain, the PAC is present when you’re not moving; then when you want to initiate a movement that PAC is strongly decreased,” she says. The neurons have to fall out of lockstep so some of them can engage in a new task. “But in Parkinson’s patients,” she says, “the neurons that are supposed to engage in the new task cannot, because they are still synchronized. So you have the difficulty with movement, the slowness, the rigidity.”
It seems likely that a similar synchronicity is at work in the brains of depressed people, says de Hemptinne, which would explain why DBS has worked for some depressed patients. “In Parkinson’s, the motor cortex is stuck in an inflexible pattern that prevents movement,” she says. “It may be the same with depression: You’re stuck in ruminative state, stuck in a inflexible pattern that prevents you from thinking differently.” De Hemptinne and her colleagues are already planning experiments to pursue this line of inquiry.
The research on Parkinson’s won’t just add to our stockpile of knowledge about the brain’s workings, it may also lead directly to the next generation of DBS devices. Experts in many neurological disorders (including epilepsy and chronic pain) are working on closed-loop systems, where an implanted device records signals from the body, and modulates its stimulation according to the patient’s need [see “Smart Neural Stimulators Listen to the Body”]. De Hemptinne says the degree of PAC in a Parkinson’s patients brain can be used to trigger the necessary bursts of stimulation.
She and colleagues have already experimented with an implant from the device manufacturer Medtronic that can both record from the brain and stimulate it. Following an initial primate study, the researchers have now moved on to the first human trials. Five people with Parkinson’s now have the Medtronic implant. “Weare starting now using it,” de Hemptinne says. “We’ll see if we can use that (PAC) signal in a closed-loop DBS.” And here’s the best part: If it works, she’ll know why.
- Toe-tapping Test for Parkinson’s - IEEE Spectrum ›
- Monitoring Parkinson's Patients at Home Could Improve Disease Management - IEEE Spectrum ›
- Deep-Brain Stimulator Draws Power from Breath - IEEE Spectrum ›
- Parkinson's Predicted From Smartwatch Data - IEEE Spectrum ›
- Spinal Stimulator's Gentle Zaps Help Treat Parkinson's - IEEE Spectrum ›
- Brain Stimulation Detoxifies the Alzheimer’s Brain - IEEE Spectrum ›
- Laser-Driven Pacemaker Guides Ailing Hearts With Light - IEEE Spectrum ›
Eliza Strickland is a senior editor at IEEE Spectrum, where she covers AI, biomedical engineering, and other topics. She holds a master’s degree in journalism from Columbia University.