What if you could plant a listening device in a single cancer cell, a bug that would follow the cell’s movements, eavesdrop on its metabolism and tell you what it’s up to?
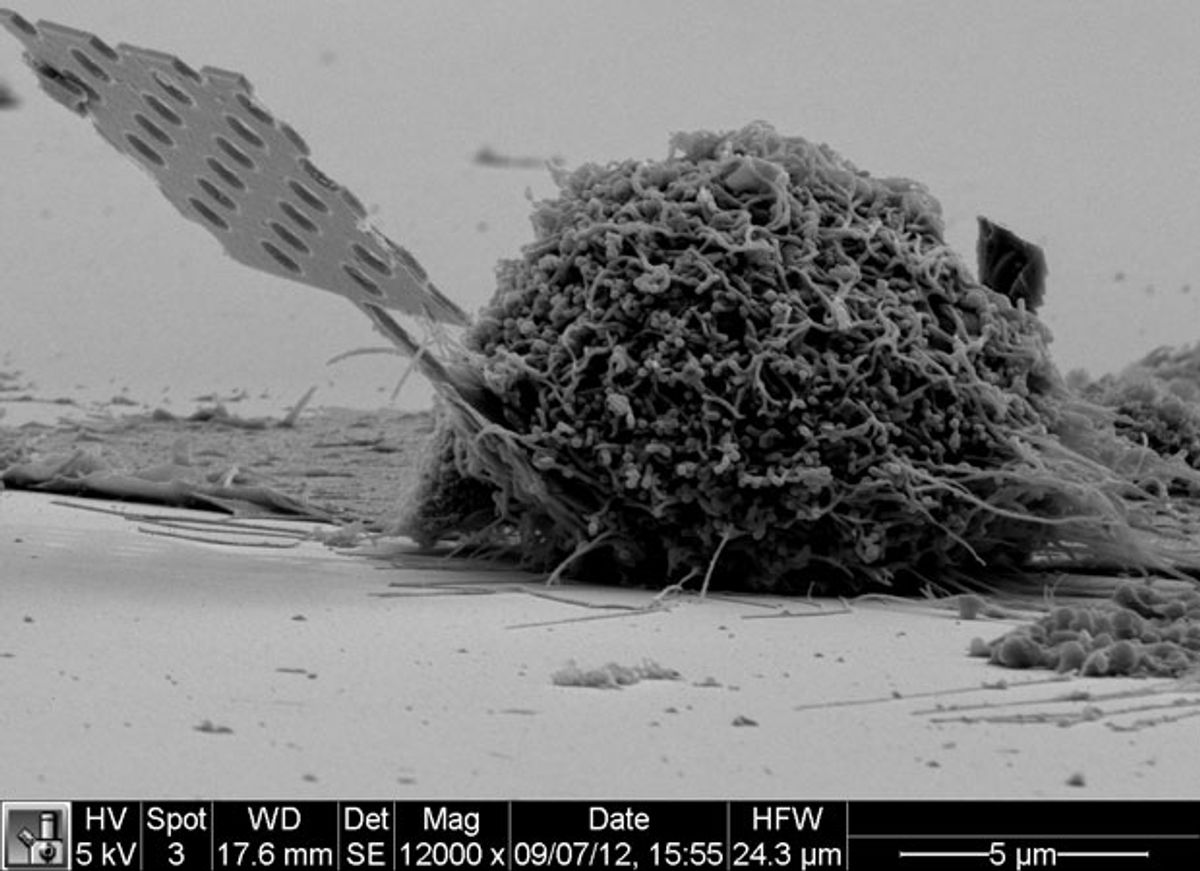
A group of Stanford University researchers has made a start with a minuscule optical-cavity splinter small enough to insert in single cells and light enough remain embedded as the cells move about and multiply.
Gary Shambat and colleagues in Jelena Vuckovic’s Nanoscale and Quantum Photonics Lab built up a gallium arsenide wafer studded with three layers of indium arsenide quantum dots. They then etched away the supporting substrate, leaving a tapering, 200-micrometer-long beam tipped with a blade 20 micrometers long, 400 to 650 nanometers wide, and 220 nanometers thick. The business end of the blade looks like a strut from an infinitesimal Meccano Erector set: it’s pierced by 20 holes (averaging 120 nm across, though their diameters and spacing diminish towards the tip); five of the holes constitute an optical cavity, a hall of mirrors that resonates at a wavelength close to the quantum dots’ 1350 nm photoluminescence.
Like so many ultra-small optical devices, the nanocavity probe’s resonant frequency changes when molecules from its environment adhere to the beam’s surface. In this case, the emitted light shifts about 6 nm to the red for every 10 nm of film thickness. In a properly constructed assay, the thickness of the film will be a function of the specific substances sticking to the probe.
Shambat, Vuckovic, and their collaborators demonstrated this phenomenon by accurately detecting the binding of streptavidin (a protein produced by Streptomyces) to biotin (vitamin B7). (The binding between the two molecules is extraordinarily strong, and serves as the foundation of countless bioassays.) The researchers coated the nanoprobe with biotin. They found that random binding of streptavidin to the probe prompted a 0.5 nm red shift, while the stronger biotin-streptavidin binding produced shifted the luminescence peak by 3.5 nm.
When further developed, the device could offer an additional tool in the increasingly important study of single living cells. “Let’s say you have a study that is interested in whether a certain drug produces or inhibits a specific protein. Our biosensor would tell definitively if the drug was working, and how well, based on the color of the light from the probe. It would be a powerful tool,” said co-author Sanjiv Sam Gambhir, chair of the Stanford Medical School radiology department.
Image: Gary Shambat/Stanford University School of Engineering
Douglas McCormick is a freelance science writer and recovering entrepreneur. He has been chief editor of Nature Biotechnology, Pharmaceutical Technology, and Biotechniques.