In 2004 two researchers, Andre Geim and Konstantin Novoselev, at the University of Manchester, in England, announced the creation of graphene, a two-dimensional form of carbon with unparalleled electronic properties. A decade earlier, Kyozaburo Takeda and Kenji Shiraishi of NTT Basic Research Laboratories, in Atsugi, Japan, predicted that a similar structure of silicon atoms—silicene—should exist. Now, a group of scientists in Europe say they’ve finally managed to create a sample of the stuff. Experts expect that silicene will have some of graphene’s amazing abilities—such as allowing electrons to speed through it as if they had no mass—but in an element more familiar to the semiconductor industry.
Geim and Novoselev had isolated graphene in an embarrassingly simple manner: They peeled it off graphite with Scotch tape. The creation of a silicene layer was much more difficult. “There is no equivalent to graphite where you could simply peel it off, and that was the problem,” says Patrick Vogt, a physicist at the Technical University of Berlin. He led a team of researchers from France and Italy, who synthesized and investigated the properties of single layers of silicene and reported the results in April’s Physical Review Letters.
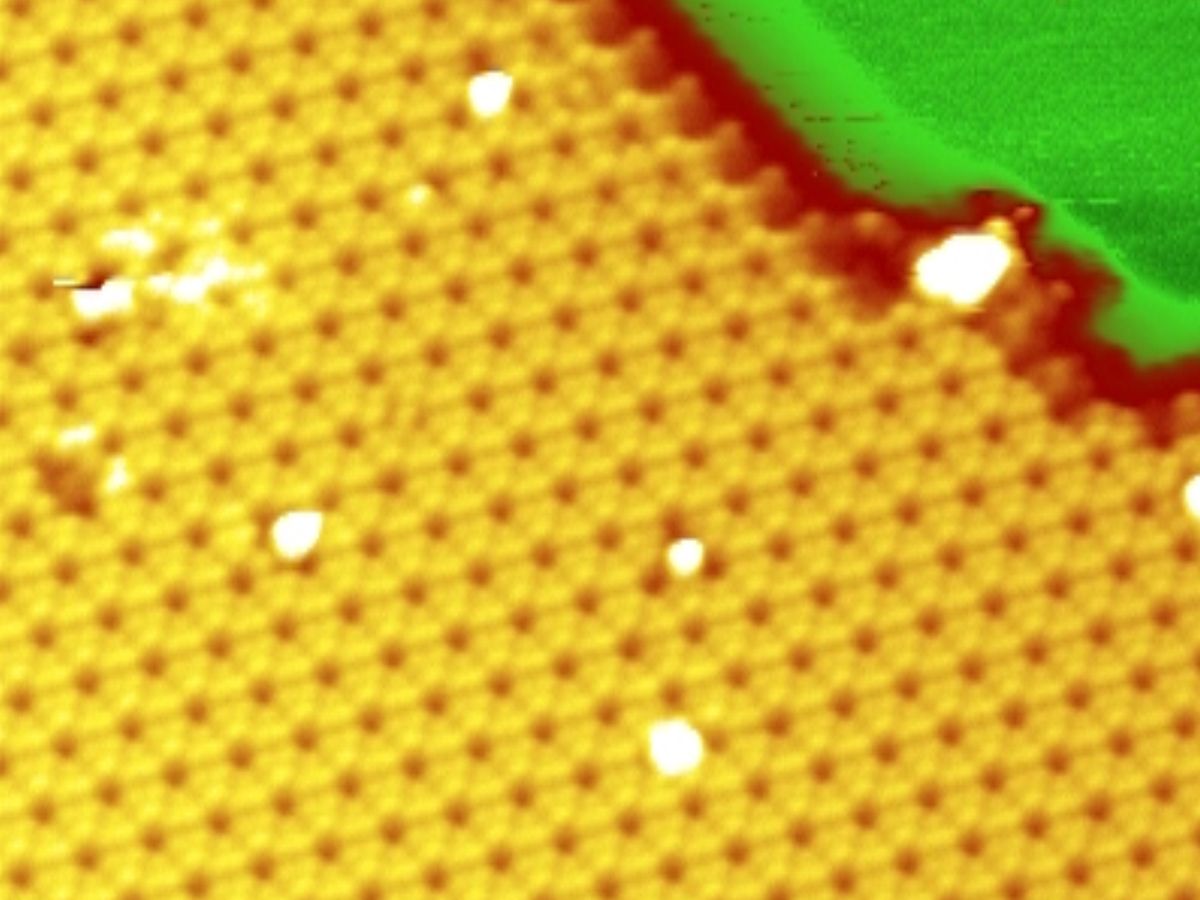
The researchers created the monolayers by evaporating away silicon from a wafer in a vacuum and allowing it to carefully deposit on a silver surface. Silicon and silver atoms do not form chemical bonds, so the silicon atoms are forced to bond with each other and settle into a honeycomb structure, says Vogt. Unlike graphene, which is perfectly planar, the silicene is corrugated.
In 2010, other researchers claimed to have made silicene. A group led by Bernard Aufray at the National Center for Scientific Research–Interdisciplinary Nanoscience Center of Marseille (CINaM), in France, found a silicene-like honeycomb structure in their scanning tunneling microscopic (STM) images following a similar evaporation experiment.
But the interatomic distances they observed were viewed by some scientists to be too small to be ascribed to silicene. And there was another possible problem, according to Vogt. “The silver surface can mimic a honey comb structure because of its interaction with the microscope,” he says.
In order to prove that they were really dealing with silicene, Vogt, along with Guy LeLay from CINaM and Paolo De Padova from CNR‑ISM in Rome, resorted to an additional experimental technique. They made STM observations of samples at different stages in the formation of the silicene layer. “We could see these silicene islands grow, and we could measure the height of the forming layer,” he says.
Just like graphene, silicene is expected to be a good conductor because electrons can travel through it quickly, as if they were massless. However, critical measurements of the electronic properties are not yet possible. One of the problems is that the silver substrate on which silicene is grown is itself a good conductor. “This makes it very difficult to understand what comes from the silver and what comes from the silicene. If we had it on a semiconductor or an insulator, that would be much easier,” says Vogt, who adds that aluminum nitride might be a possible substrate, an idea he plans to investigate.
For his part, Aufray intends to try to deposit silicene on sodium chloride surfaces, which have a crystalline structure close to that of silver. And his group claims in the April issue of Journal of Physics: Condensed Matter that its original work showed silicene as well. Aufray even argues that the layer obtained by Vogt and his team might not be a real silicon equivalent of graphene. “We think that silver also plays a role as a catalyst and could force the silicon atoms in specific positions,” says Aufray.
So the jury is still out on whether anyone has truly created a silicon analogue to graphene. And if they have, would it be useful in electronics?
Walter de Heer, who studies graphene at Georgia Tech, thinks it is unlikely because silicene cannot exist on its own without a support. “It deforms very easily and turns into a drop of silicon,” he says. “As compared to graphene, silicene is quite unstable.”
However, some graphene researchers are open to the possibility. “Just because 3-D silicon is the most important material available today, we shouldn’t jump to the conclusion that 2-D silicon will be equally important,” says Aravind Vijayaraghavan, a graphene researcher at the University of Manchester. “On the other hand, it might turn out to be even better!”