Nearly three decades ago, our understanding that there were three basic forms of carbon—diamond, graphite, and amorphous carbon—was stood on its head with the introduction of fullerenes, then carbon nanotubes, and more recently graphene.
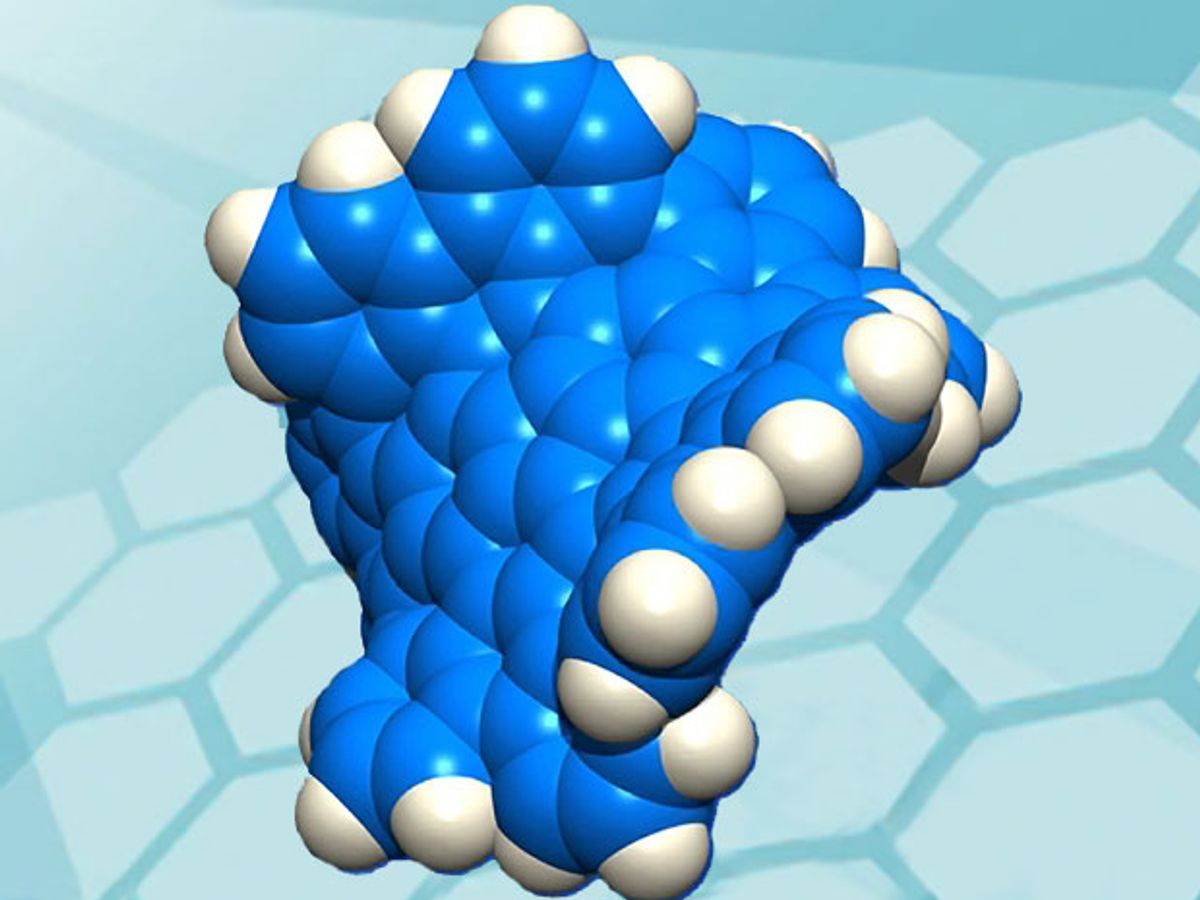
Now researchers at Boston College and Nagoya University have synthesized another new form of carbon unofficially dubbed “grossly warped nanographenes.” The research, which was published in the journal Nature Chemistry (“A grossly warped nanographene and the consequences of multiple odd-membered-ring defects”), has led to creating a material that is essentially defects in the two-dimensional hexagonal honeycomb-like arrangements of trigonal carbon atoms found in graphene. These defects consist of non-hexagonal rings that force distortions out of the two-dimensional plane.
The grossly warped nanographene consists of 80 carbon atoms joined together in a network of 26 rings, with 30 hydrogen atoms on the outside rim. In contrast to graphene sheets, which typically have planar two-dimensional geometries, the new material juts out from a single plane because of the five 7-membered rings and one 5-membered ring embedded in the hexagonal lattice of carbon atoms.
Pushing its geometry out of planarity has altered the new material's physical, optical, and electronic properties vis-à-vis its carbon cousins.
“Our new grossly warped nanographene is dramatically more soluble than a planar nanographene of comparable size,” said Lawrence T. Scott, professor at Boston College and one of the principal authors of the research, in a press release. “The two differ significantly in color, as well. Electrochemical measurements revealed that the planar and the warped nanographenes are equally easily oxidized, but the warped nanographene is more difficult to reduce.”
These are just the initial differentiating characteristics of this new form of carbon. If the near-decade-long history of graphene is any guide, we can expect new properties to be discovered on a regular basis. (Just this week, for example, researchers discovered in measurements that the response rate of an optical switch using graphene is 100 times faster than traditional materials.)
Whether the so-called “grossly warped nanographenes” will offer the same wealth of new characteristics as graphene remains to be seen. However, it would seem that graphene is now not only facing competition from other 2-D materials, but from an entirely new form of carbon.
Illustration: Boston College
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.