Researchers at Michigan Technological University (MTU) believe they have developed a new approach that will give us the best of both worlds: the quick power bursts for which capacitors are known and the long run times we get from electrochemical batteries.
While nanotechnology has had a big impact on ultracapacitors (sometimes referred to as supercapacitors) for some time now, getting ultracapacitors to behave more like batteries has remained an important research focus. Ultracapacitors are still largely relegated to applications where quick bursts of energy are needed (this is often referred to as power density, or the ability to carry large amounts of current out of the capacitor). Applications that need long runtimes of continuous energy (often referred to as energy density) has continued to be the domain of electrochemical batteries.
The MTU researchers, led by Dennis Desheng Meng, were led to their new reduction-oxidation (redox) capacitor by reevaluating the long promising but often disappointing material, manganese dioxide. Manganese dioxide has been attractive in this application because it’s abundant and environmentally friendly. But it didn’t provide the power density of carbon-based physical capacitors.
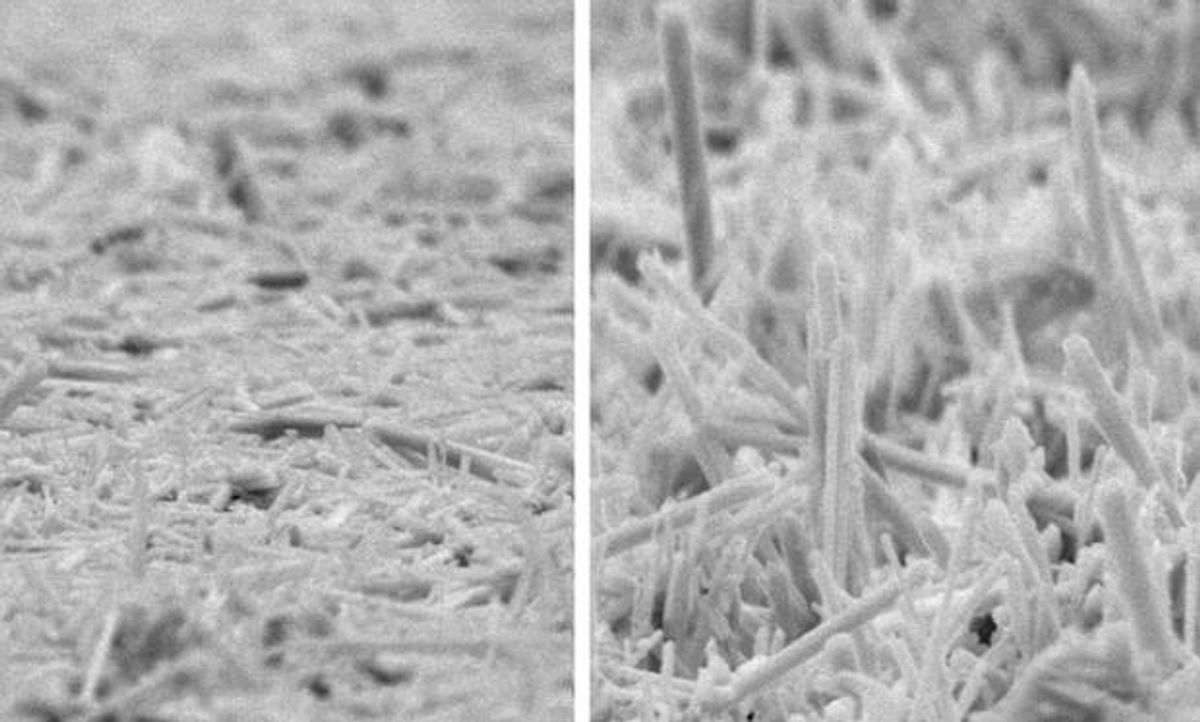
Meng believed that if the manganese dioxide could be used in the form of nanorods then the material could possess the right attributes for it to compete with carbon.
Of course, he's not the first to try to use manganese dioxide nanorods for generating electricity. In one notable example, Yi Cui, Associate Professor of Materials Science and Engineering at Stanford University, had some success in using manganese dioxide in nanorod form in a technique known as pressure-retarded osmosis in which the difference in salinity between freshwater and saltwater can be exploited to generate electricity.
Meng’s application of the manganese dioxide nanorods is quite a bit different and as such the material needed to have some very different qualities. The nanorods needed to possess both an ideal crystalline structure and be aligned, two qualities that until now had remained mutually exclusive.
In the MTU research, which was published in the journal ACS Nano (“Scalable High-Power Redox Capacitors with Aligned Nanoforests of Crystalline MnO2 Nanorods by High Voltage Electrophoretic Deposition”), Meng was able to give the nanorods both of these qualities through electrophoretic deposition in which nanoparticles are deposited on a substrate in the presence of an electrical field. This manufacturing technique produced nanorods that aligned themselves like trees in a forest—upright and tall—and still possessed the ideal crystalline structure.
This combination of features—ideal crystalline structures that were all in alignment—minimized internal resistance within the capacitor, permitting the capacitor to charge and discharge repeatedly without much wear. In fact, the researchers were able to go through 2000 charge/discharge cycles on their prototype capacitor and still maintain over 90 percent of its original charge.
Perhaps the most attractive aspect of the manufacturing technique is its scalability. “We did it in a lab, but this is scalable manufacturing,” says Meng in a press release. “We can continuously print it out in a roll-to-roll manner, and you can make the substrate very large if you like.”
Meng foresees this kind of nanocapacitor coupled with solar cells or used in hybrid electric vehicles, where it could help with quicker accelerations.
Photo: Sunand Santhanagopalan
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.