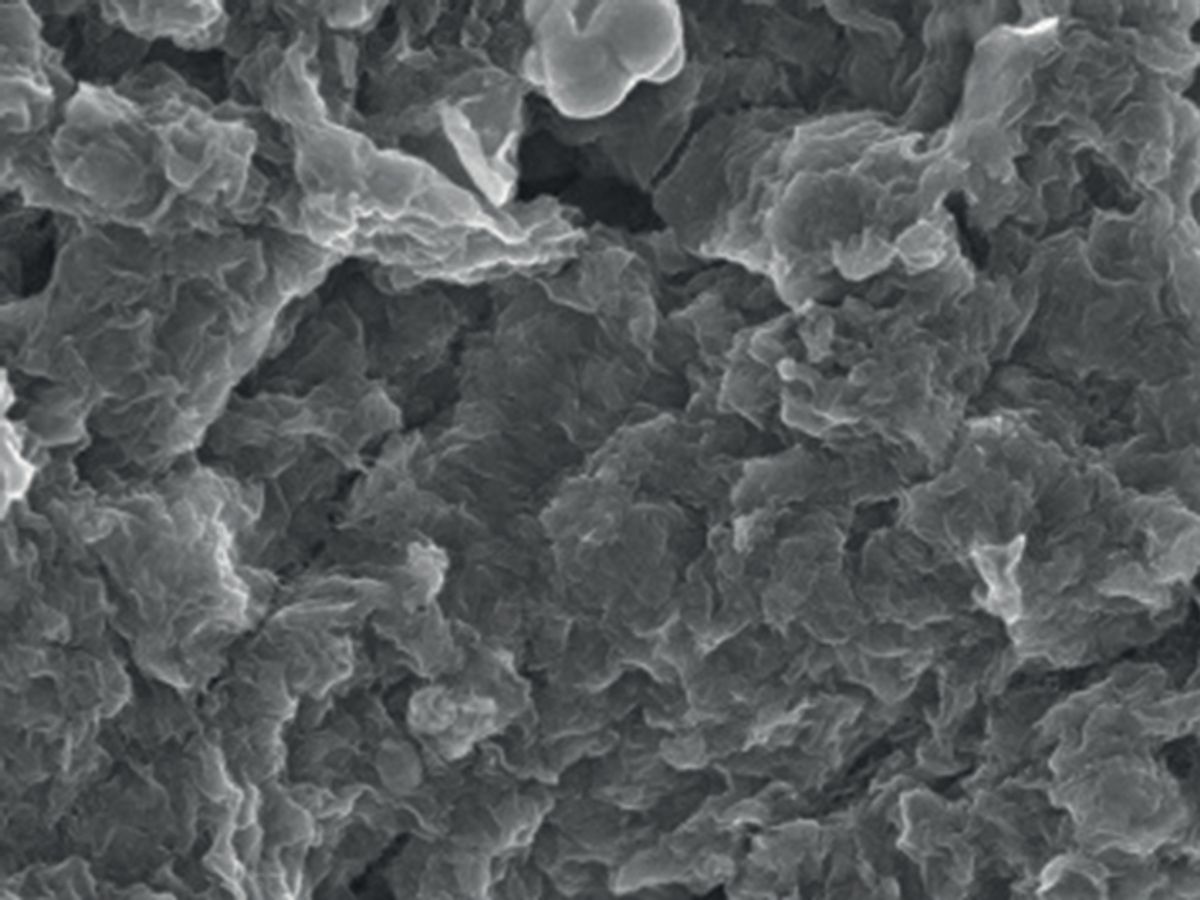
Researchers at the U.S. Department of Energy’s (DOE’s) Argonne National Laboratory have added a twist to lithium-oxygen batteries (Li–O2) by successfully demonstrating for the first time the ability to stabilize crystalline lithium-superoxide (LiO2) which could usher in an entirely new class of batteries.
In research published in the journal Nature, the Argonne researchers were able to produce the stable lithium-superoxide by using a graphene-based cathode. This approach avoids the presence of lithium peroxide (Li2O2), a solid precipitate that clogs the pores of the electrode in lithium-oxygen (aka lithium-air) batteries.
Lithium-air batteries use the oxidation of lithium at the anode of the battery and the reduction of oxygen at the cathode to create a current. This battery design has promised up to 10 times as much energy as a lithium-ion battery at the same weight, giving them an energy density equal to that of gasoline.
While other researchers, like those at the University of Cambridge, have successfully used graphene-based cathodes to avoid the production of lithium peroxide particles, the Argonne team’s work stands apart thanks to a new template growth mechanism for the crystalline lithium-superoxide based on the use of iridium nanoparticles.
Both Larry Curtiss and Khalil Amine, two of the Argonne researchers, credited the growth of the lithium superoxide to the spacing of the iridium atoms in the electrode and believe that iridium will serve as a useful template for the growth of the superoxide going into the future.
The key advantage of a battery based on superoxide, according to the two researchers, is that it enables what is known as a “closed system,” which stands in contrast to open systems that require a constant intake of oxygen from the environment. This makes closed system batteries far safer and more efficient.
"This discovery really opens a pathway for the potential development of a new kind of battery," said Curtiss, one of the researchers, in a press release. "Although a lot more research is needed, the cycle life of the battery is what we were looking for."
Amine, added: "The stabilization of the superoxide phase could lead to developing a new closed battery system based on lithium superoxide, which has the potential of offering truly five times the energy density of lithium ion.”
This much energy density is the key factor in pursuing lithium-oxygen (also known as lithium-air) batteries over lithium-ion (Li-ion) batteries, especially for all-electric-vehicle applications. Following the demise of a few high-profile nanotech-companies that aimed to bring a new and improved Li-ion battery to the all-electric vehicle market, it began to sink in with the research community that perhaps the Li-ion battery was not the answer for this particular application.
It was about this time in late 2012 that there was a sudden uptick in research looking into the potential of so-called lithium-air batteries. If this work out of Argonne and the previous work out the University of Cambridge are any indication, there’s real potential for lithium-air batteries to someday power our vehicles and give us the same bang as fossil fuels.
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.