Vanadium dioxide (VO2) has both fascinated and vexed researchers ever since it was discovered at Bell Laboratories back in 1959. Because of strong interactions between its electrons, it is one of the few known materials that can switch rapidly between being an electrical insulator and a conductor—a phenomenon known as metal-insulator transition.
This quick change ability led to the material being used in devices such the first Mott transistor. But recently, vanadium seemed to lose some of the luster that came from anticipation of it being the next-generation transistor material. While it could still eventually be that wonder material, it was revealed that physicists knew a lot less about how it operated than they had previously thought.
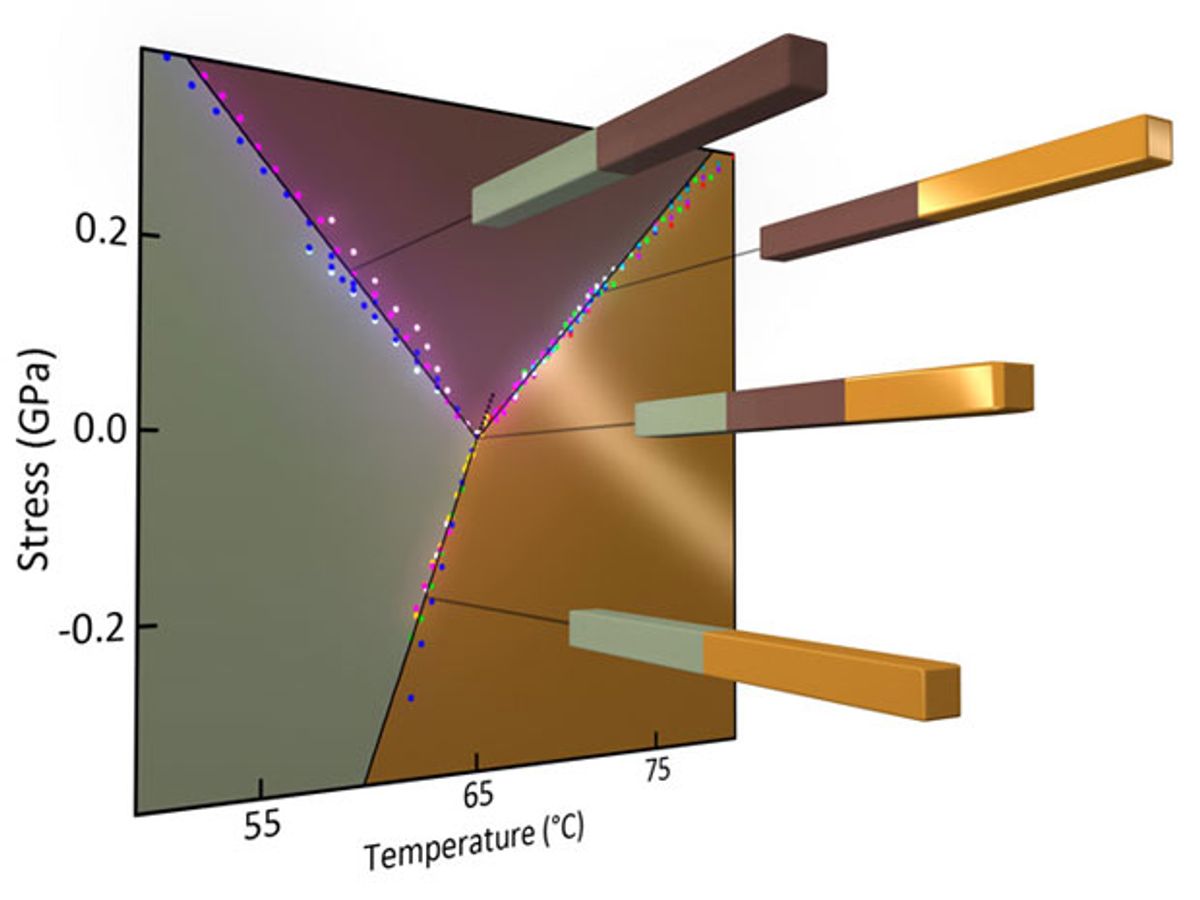
To overcome at least some of that knowledge gap, researchers at the University of Washington have observed the exact point at which three solid phases of VO2—the so-called triple point—can coexist stably.
The research, which was published in the journal Nature (“Measurement of a solid-state triple point at the metal–insulator transition in VO2”), represents the first time that a triple point has been accurately determined for any substance.
“These solid-state triple points are fiendishly difficult to study, essentially because the different shapes of the solid phases makes it hard for them to match up happily at their interfaces,” said David Cobden, a University of Washington physics professor, in a press release.
It has been known for the last 30 years that VO2 has two slightly different insulator phases. The University of Washington research revealed that these two phases and its conducting phase can coexist stably at 65 degrees Celsius (149 °F).
Cobden and his colleagues initially tried to pinpoint this temperature by pulling at VO2 nanowires while observing them under a microscope. But as it so happened, VO2 surprised the researchers: The triple point revealed itself when the nanowires were neither pulled nor compressed but when they were left alone.
After spending years developing the apparatus for conducting the experiments, and then carrying them out, the researchers began to realize that VO2’s triple point was key to its fascinating phase transition properties.
“If you don’t know the triple point, you don’t know the basic facts about this phase transition,” Cobden says in the release. “You will never be able to make use of the transition unless you understand it better.”
Whether this understanding will win back some of luster that the material has lost of late remains to be seen. But this work does open the possibility that researchers' understanding of vanadium's properties will yield tangible benefits in the future.
Image: David Cobden/University of Washington
Dexter Johnson is a contributing editor at IEEE Spectrum, with a focus on nanotechnology.