Platinum is widely used as a catalyst for oxygen reduction reactions in fuel cells, but its high cost is a major obstacle to making fuel cell vehicles more affordable and more popular. Graphene, however, may be just what automotive and energy companies are looking for.
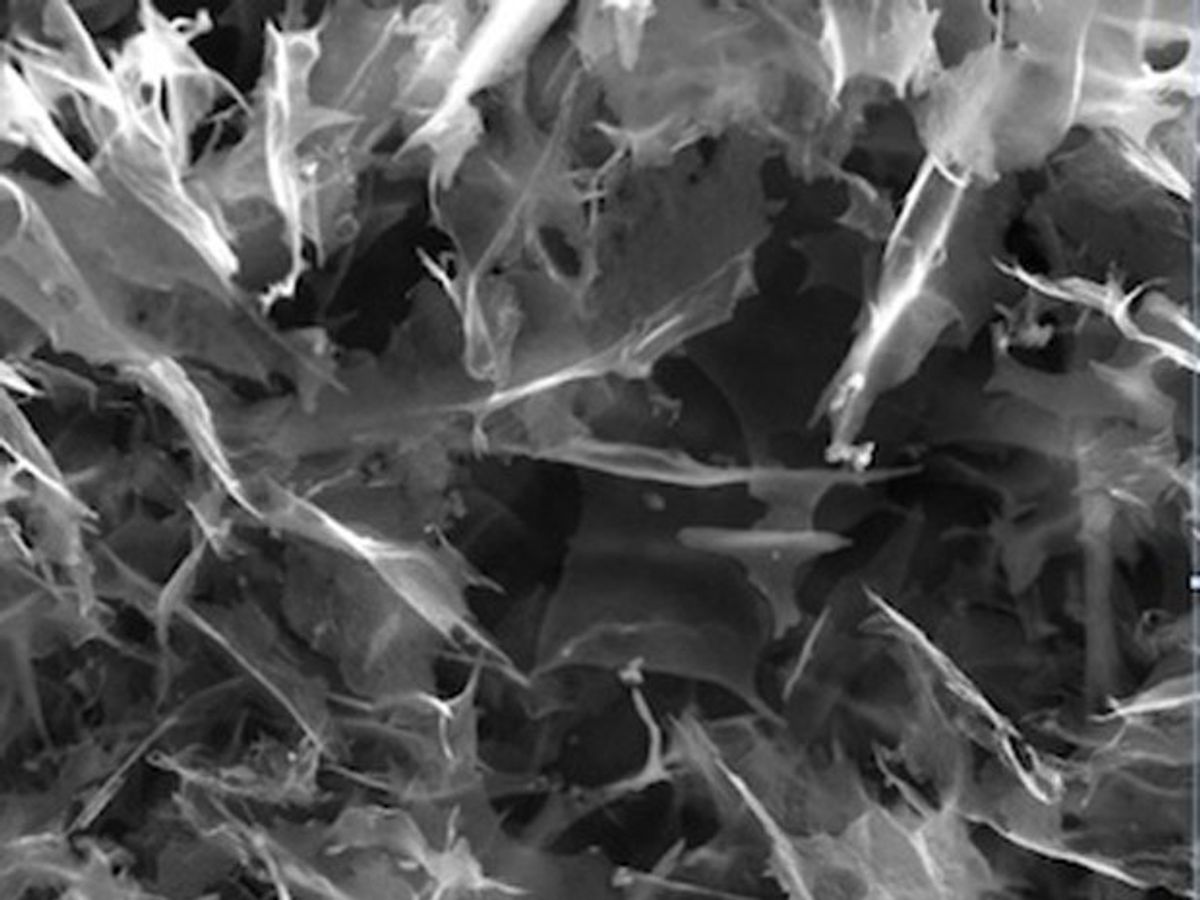
Researchers from Rice University attached graphene quantum dots to a graphene base, resulting in a hybrid material that operates as an excellent– and cheap–catalyst for fuel cell reactions.
Quantum dots are nanocrystals that exhibit quantum mechanical properties, making them very useful in experimental transistors, solar cells, imaging chips, and other things. James Tour, a Rice chemistry professor, and his colleagues created graphene quantum dots (GQDs) from coal last year, and followed up on that breakthrough with this latest experiment.
This time, those same GQDs were combined with microscopic sheets of graphene into self-assembling nanoscale platelets. Tour explains that in the hybrid GQD/graphene material, the quantum dots provide a high abundance of edges where chemical reactions can occur, while the graphene is a plane of conductivity between GQDs. (The material was also treated with boron and nitrogen–co-catalysts.)
The lab discovered that the new material actually outperforms platinum-based catalysts for fuel cell reactions. Compared to platinum, the GQD/graphene material showed an oxygen reduction reaction with about 15 millivolts more in positive onset potential, or the start of the reaction. “You don’t need to apply as high a voltage as platinum to get the oxygen reduction reaction to occur,” says Tour. “We also get about 70 percent higher current than what platinum would offer.”
Increased efficiency aside, the hybrid material is also cheaper to make and install in fuel cells. The average cost of a fuel cell for an automobile has gone down from $275 per kW capability in 2002, to $55 per kW in 2013. Yet the fuel cell industry has yet to make a profit. The U.S. Department of Energy believes automotive fuel cell costs will need to fall to $30 per kW before we can expect to see real consumer interest. Toyota began selling its hybrid hydrogen fuel cell car in Japan this year for around $68,600. Unfortunately, with a 2014 Corolla going for between $16,000 and $21,000, fuel cell vehicles have yet to be noticed by most consumers.
The researchers believe the new findings, published in ACS Nano, could pave the way toward that goal. “This allows one of those two key reactions in the fuel cell to happen very well for the formation of water,” says Tour. “With fuel cells, the concern is always the cost of platinum electrode. This obviously needs no platinum electrode.”
However, Tour is very hesitant to say the fuel cell era is just over the horizon, and with good reason. “We don’t have an infrastructure as a country or global system for exploiting hydrogen-based systems well. You have to have a buy-in from everyone involved in that system for things to start taking off.”