Metal Fuel Cells
For everything from EVs to backup generators, they may provide practical and efficient energy storage
Like many recent visitors to China, Sadeg Faris was appalled by the quality of the air in the big cities. "In Beijing," he said, "the air was so terrible, I couldn't see the sun."
Unlike most visitors to the Middle Kingdom, however, Faris is in a position to do something about that situation. His company, eVonyx Inc. (so spelled to reflect its intended pronunciation, ee-vee-onyx), Hawthorne, N.Y., is developing metal-based fuel cells that Faris hopes will one day help improve air quality by powering electric cars and trucks in crowded cities around the world.
To get his plan moving, though, Faris will begin in a more modest way: by making small fuel cell systems for powering electric scooters in Southeast Asia [figure]. If successful, these fuel cells could go a long way toward cleaning up the air in that part of the world, where scooters and motorbikes powered by highly polluting two-stroke engines are the most common form of motorized transport.
In choosing electric vehicles as the principal application of his fuel cells, Faris is bucking a trend. Most fuel-cell manufacturers agree that while EVs will eventually be an excellent application for fuel cells, that market will not materialize for years.
A more immediate application for metal-based fuel cells is as backup and emergency electricity sources to replace generators driven by internal combustion engines. As Jeff Colborn, president and chief executive officer of Metallic Power Inc., Carlsbad, Calif., sees it, one of his company's zinc-air fuel cell systems could sit in an office during a power outage, powering computers, lights, and other gear while generating neither noise nor pollutants.
Another maker of metal-based cells, Aluminum-Power Inc., Toronto, sees backup power systems for telecommunications centers as the most immediately profitable use for its products. The key application, according to Rafael Ferry, Aluminum-Power's marketing vice president, will be in metropolitan-area networks, where it is very expensive to rent space for conventional lead-acid batteries. Fuel cells have much higher volumetric energy densities than those batteries and therefore require much less real estate to provide the same amount of backup.
Although these executives don't agree on all the details in applying this technology, they do have a common belief: better fuel cells can be made by basing them on metals like aluminum or zinc instead of on hydrogen gas. So far, all three companies are still in the prototype stage of fuel-cell development, with commercial products a year or so away. Those products will include small battery-like units suitable for powering cell phones and laptop computers, medium-sized (around 5-kW) portable systems for emergency use, and large (on the order of 1-MW) stationary systems.
Hydrogen headaches
The best-known fuel cells, the ones based on hydrogen, feed a continuous stream of the gas to the anode [see "Fuel Cells for Dummies"]. The waste product is water vapor, which can be freely discharged into the atmosphere and is recycled for free by Mother Nature, a neat advantage. Such cells are being deployed today on a more-or-less experimental basis in vehicles, especially buses, and in stationary applications.
Another plus for these hydrogen-based systems (and others based on flowing gases or liquids) is easy thermal management--no need to add a separate liquid or air heating or cooling system. On a weight basis, too, hydrogen is a very efficient fuel; each kilogram of it packs 42 kWh of energy--three times as much as the same weight of gasoline and a thousand times as much as a lead-acid battery of the same weight.
In the near-term, metal-based fuel cells could replace backup generators driven by internal combustion engines
So why all the interest in metal-based fuel cells? Briefly stated, they store a lot of energy in a small space both conveniently and safely.
Although hydrogen does indeed have a specific energy of 42 kWh/kg, it is such a light substance that squeezing a kilogram of it into a reasonable amount of space presents quite a challenge. At the not inconsiderable pressure of 35 megapascals (over 345 atmospheres), a liter of hydrogen weighs only 31 grams and contains 1.3 kWh of energy. The result is that the containment vessel weighs considerably more than the gas.
A liter of solid aluminum, in contrast, weighs 2.7 kg and can theoretically yield over 10 times as much energy. Zinc has a lower specific energy than aluminum, but because it is denser, it winds up packing almost as much energy as the lighter metal into the same amount of space.
The problem with hydrogen is that, to be a practical fuel, it must be stored and transported under pressure. That stipulation raises a host of convenience and safety issues, not the least of which is the need for government permits to store and transport it. One thing that everyone involved with alternative energy sources seems to agree on is that building a refueling or recharging infrastructure is a major--perhaps the major--difficulty to be overcome. These experts point out that if gasoline were not already established as an irreplaceable part of modern life, it would probably never be approved as a fuel in today's regulatory environment. Hydrogen, although not nearly as dangerous, has scared people ever since the Hindenburg airship disaster more than half a century ago. When hydrogen leaks, the gas tends to rise and dissipate, unlike heavy gasoline vapors, which tend to gather in low places and wait for unsuspecting victims to touch them off.
Aluminum and zinc, in contrast, are benign materials that are also perceived to be benign. They can be transported without restriction and stored just about anywhere. True, fuel cells based on these metals do produce wastes that cannot simply be dispersed into the atmosphere, but the waste products--aluminum oxide and zinc oxide--are widely recognized as nonflammable, nontoxic materials that present no shipping or storage problems. And, they can be easily recycled.
Size counts
As comedians and venture capitalists are fond of pointing out, timing is everything. For the developers of metal fuel cells, it's unfortunate that they weren't a year or so further along in their product development when the California energy crisis hit early this year. If they had been, they would have had a chance to demonstrate the capabilities of their products and to share in the profits made by makers of gasoline-powered emergency generators. Whether the crisis will oblige them and stick around long enough (or even spread) to help them is hard to foresee.
Energy crisis or no energy crisis, however, one market that will always need backup power sources is telecommunications. Telecom central offices use a lot of power, and standard practice dictates that they be able to operate for 8 hours in a power emergency. Traditionally, lead-acid batteries have provided the backup. They do not store much energy per kilogram, but since they usually just sit on a basement floor, weight is no big problem.
What is a problem, though, is that lead batteries store little energy per unit volume. Given the cost of real estate in metropolitan areas, what counts in metropolitan-area networks, therefore, is size--that is, volumetric energy density--usually expressed in watt-hours per liter. According to Aluminum-Power's Ferry, conventional lead-acid batteries, of the kind now in widespread use for telecommunications backup, store 2040 Wh/L. The aluminum fuel cells his company is currently developing do about 10 times as well.
Still, aluminum is not without problems of its own. A big one is parasitic corrosion. Leave aluminum plates in contact with a caustic solution, like the electrolytes used in fuel cells, and they will rapidly disappear, losing a few percent of their mass per hour. Aluminum-Power's solution to this problem is to separate the plates from the potassium hydroxide electrolyte. The company's backup fuel cell systems keep the caustic alkaline solution in a separate tank and pump it into the stack only when it's time to deliver power.
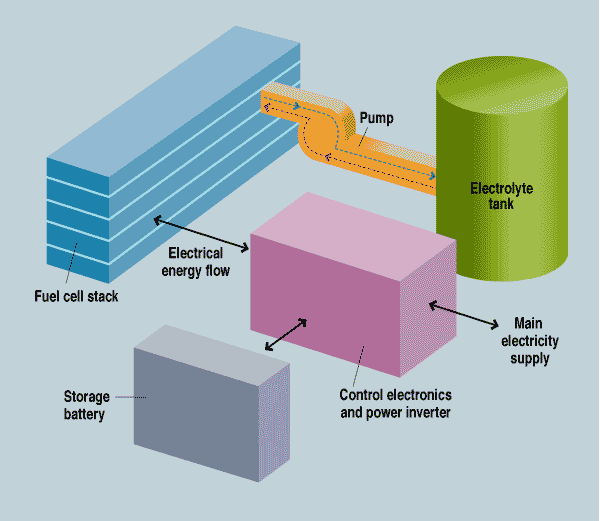
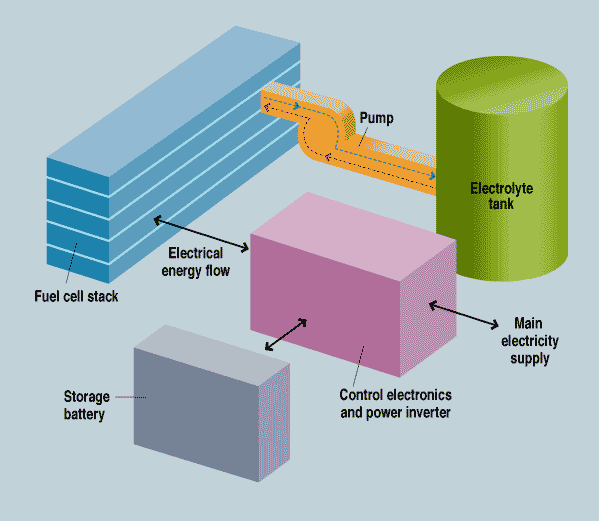
The system has five main components: the fuel cell stack, the electrolyte tank, a pump, a storage battery, and control circuitry [see illustration]. In case of a power failure, the battery provides instantaneous backup and also powers the pump, which transfers the electrolyte from its storage tank into the stack. Once that transfer is made, the stack immediately begins generating electricity, providing backup power and recharging the battery. When the emergency is over, the pump removes the electrolyte from the stack, shutting down the fuel cell and preventing parasitic corrosion. When the aluminum plates in the stack are depleted (or any time before that, if desired), the cell is refueled by simply replacing them.
Interestingly, Aluminum-Power has also developed very small aluminum fuel cells suitable for cell phones. These units, of course, cannot encompass storage tanks and pumps. Instead, they rely on proprietary formulations for both the anode and the electrolyte that reduce (but do not eliminate) corrosion. The cells are refueled by simply inserting a fresh aluminum anode when necessary. To facilitate anode replacement, the electrolyte is in the form of a gel with proprietary additives that cause it to crystallize and fall away from the anode reaction surface.
Aluminum-Power recently licensed its small-cell technology to Trimol Group Inc., New York City, which will pursue applications in cell phones and, later, in laptop computers. Aluminum-Power itself intends to focus on larger applications: backup systems now and electric vehicles later.
Is zinc the answer?
Another way to deal with the problems of aluminum being corroded by its electrolyte is to avoid that metal altogether and use zinc. Although theoretically able to store only a quarter as much energy per unit mass as aluminum (around 4 kWh/kg for aluminum compared with 1 kWh/kg for zinc), zinc can be used to make quite competitive fuel cells. Practical zinc-air fuel cells can be made to achieve ratings of about 350500 Wh/kg compared with perhaps 800 Wh/kg for aluminum, according to Jeff Colborn of Metallic Power, who spent several years trying to overcome the lighter metal's corrosion problems but finally gave up and switched to zinc.
Unlike aluminum, zinc will corrode only slowly when immersed in its alkaline electrolyte, which is also potassium hydroxide. To keep a zinc-air fuel cell from losing capacity while in standby mode, it is only necessary to shut off its air supply. That, obviously, is easier to do than to separate it from the electrolyte.
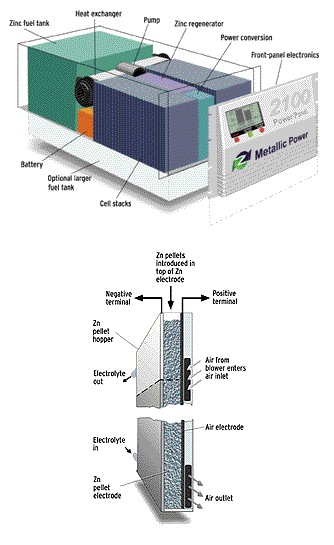
Taking advantage of the fact that zinc can be left in contact with a corrosive electrolyte, Metallic Power has come up with a unique refueling approach that is especially well suited to electric vehicles. The "spent" fuel (zinc oxide), along with some of the liquid electrolyte solution, is pumped from the fuel cell into a vending-machinesized refueling unit. At the same time, zinc pellets, about a millimeter across, are pumped into the fuel cell along with replacement liquid electrolyte from the same refueler.
The bonus is that the refueling unit also includes a regenerator that recycles the zinc oxide back into zinc. Using electricity from the grid, the unit converts the zinc oxide back into metallic zinc granules and releases oxygen into the atmosphere. It is, Colborn told IEEE Spectrum, "a completely closed-loop system with nothing to add, nothing to discard, and nothing wasted."
For stationary backup applications, Metallic Power has developed "hot-swappable" cartridges containing the zinc-electrolyte mixture. These cartridges, which can be swapped without shutting down the system, lend themselves to several scenarios. Spent ones can be exchanged for fresh ones at a recycling center; picked up by a service provider, who also delivers fresh ones; or regenerated by the user when grid power becomes available. For this last approach, the company makes a fuel cell system with a built-in regenerator [figure].
A crucial aspect of the Metallic Power approach is that the bed of zinc granules in each cell is continuously washed by a recirculating flow of electrolyte [opposite page, bottom]. That not only keeps the stack cool, it also removes the intermediate soluble zincate reaction product, thereby reducing the precipitation of zinc oxide, the final--insoluble--reaction product, in the active electrode area. Without this feature, the cell would quickly become clogged with a zinc oxide mixture that would make it unrefuelable.
Assessing the prospects of metal fuel cells, with their potassium hydroxide electrolytes, H. Frank Gibbard, chief executive officer at H Power Corp., a Clifton, N.J., manufacturer of hydrogen fuel cells, points out that KOH is a nasty material to handle, with a propensity to climb up and over the rims of containers in which it is stored. It is one thing, he told Spectrum, to make a small alkaline cell with a rather short seal, but it is quite another to engineer the larger components needed for fuel cells.
Metallic Power's Jeff Colborn agrees that the issue is not trivial, but asserts that if companies have any difficulties designing the necessary seals, it is because the wrong people--namely, electrochemists-- tend to work on the problem. He's assigned mechanical engineers to the task, people with experience at oil companies, where they learned to build systems capable of handling high-pressure steam mixed with combinations of petroleum distillates and even sand. They have had no trouble keeping the electrolyte where it belongs, he said.
How dry it is
An even better way to avoid sealing problems with liquids, of course, is to refrain from using liquids in the first place. That's what Sadeg Faris is trying to do at eVonyx, although he has admittedly not yet reached that goal. Clearly Faris is a man who enjoys a challenge. Not only is he attempting to make a dry aluminum fuel cell, but he is concentrating on the electric vehicle market, with its formidable infrastructure and marketing challenges. The company's research is based on so-called "dry" electrolytes--that is, thin membranes that contain some moisture, but are not palpably wet--and whose operation involves no pumps or flowing electrolyte.
Mindful of the need to generate revenue while pursuing the main objective, eVonyx is developing several "easier" product possibilities while continuing research on aluminum fuel cells. These possibilities include both zinc- and aluminum-based fuel cells that use gelled liquid electrolytes.
During Faris's trip to the Far East, he arranged for the leasing of a 4000-m2 facility in Taiwan for the manufacture of zinc-air fuel cells to power electric scooters. These cells will use gelled electrolytes, and as such will represent an interim technology, as far as Faris is concerned.
His goal is to perfect an aluminum fuel cell based on a three-layer laminate of aluminum, membrane electrolyte, and air cathode that can be cheaply produced in large quantities [see Technology Watch, November 2000, p. 32]. If he succeeds, he will have a good chance of leading the way to powering electric vehicles since a dry aluminum fuel cell would be quite attractive to users and would require no regulatory approval. But that's a big IF.
To Probe Further
A good textbook on fuel cells in general is Fuel Cell Systems Explained by James Larminie and Andrew Dicks (John Wiley & Sons, Chichester, UK, 2000). It deals with many kinds of these devices and devotes one chapter exclusively to proton exchange membrane (PEM) fuel cells. The book covers, besides the cells themselves, related equipment like dc-to-ac inverters.
The companies mentioned in the article all have Web sites.