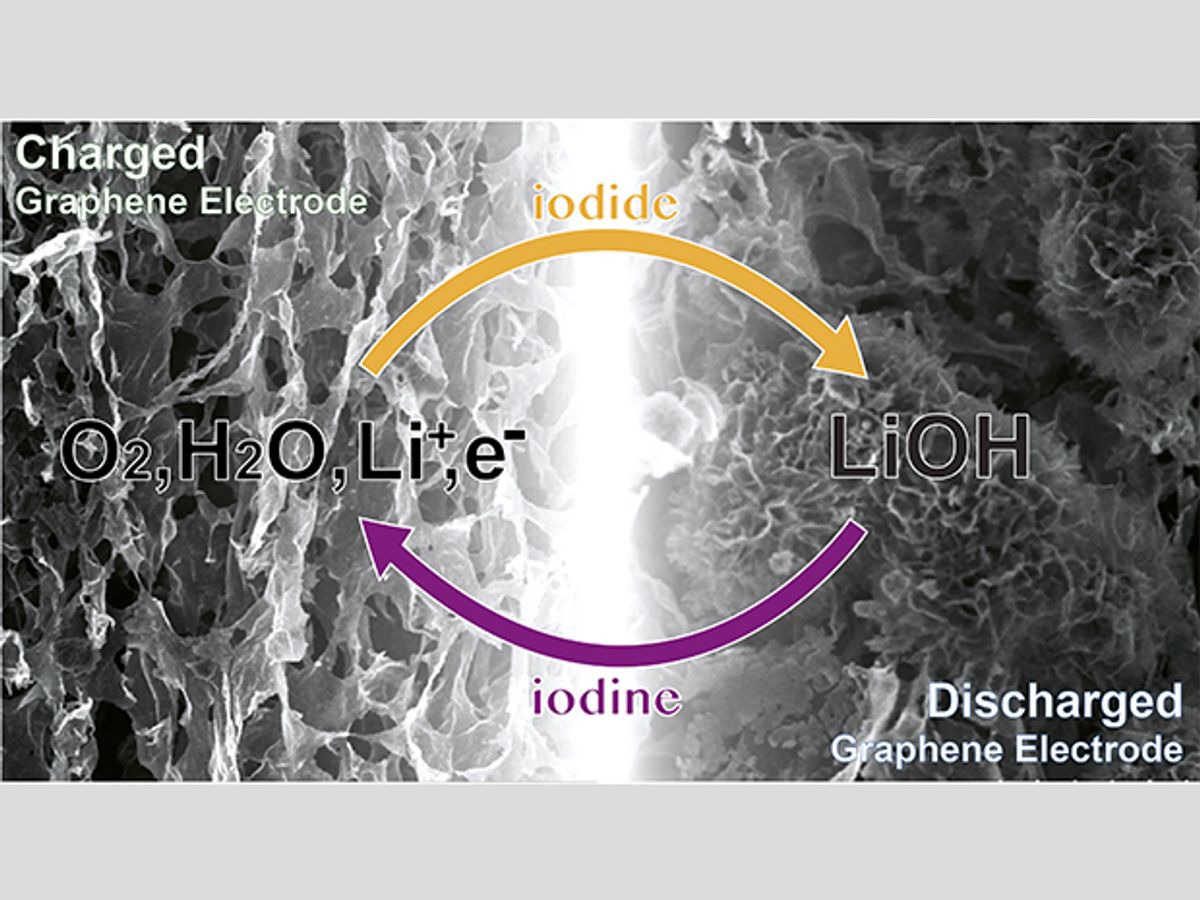
Lithium-air batteries can in principle hold five to 10 times as much energy as a lithium-ion battery of the same weight and double the amount for the same volume. They could theoretically give electric cars the same range as gasoline ones. Now scientists in England claim to have overcome many of the current barriers preventing their use.
In a lithium-air battery, the anode is generally made of lithium metal, the cathode is typically a porous carbon material that brings in oxygen from the surrounding air, and the electrolyte is a liquid connecting the anode and the cathode, helping ions shuffle between them. As the lithium oxidizes, it discharges electricity. Recharging the device reverses the process.
A number of major pitfalls have kept lithium-air batteries from meeting their full potential. For instance, when they discharge electricity, lithium peroxide particles usually form that can clog the porous cathodes, stifling the batteries. In addition, unwanted chemical reactions can attack the electrolytes, reducing the battery's overall efficiency. Moreover, these devices are very sensitive to moisture in the air, which can corrode the lithium metal anode.
To overcome these problems, scientists at the University of Cambridge designed their battery to produce lithium hydroxide crystals instead of lithium peroxide particles, and added lithium iodide as a mediator compound. These changes greatly reduced unwanted chemical reactions that can damage the electrolytes of lithium-air batteries and kill the devices.
Moreover, the researchers used macroporous reduced graphene oxide for their cathode instead of the mesoporous carbon typically used in other lithium-air battery cathodes. This thousand-fold increase in pore size — from pores 10 to 100 nanometers wide to ones 10 to 100 micrometers wide — helped prevent the lithium hydroxide crystals that formed during battery operations from clogging the pores. The scientists introduced the solvent dimethoxyethane to help remove these crystals as the battery charged and discharged.
Furthermore, this new design showed a high tolerance for water, suggesting it could tolerate moisture in the air. "This finding of water tolerance is another step forward towards a practical lithium-air battery," says study lead author Tao Liu at the University of Cambridge.
Future research can explore bringing more oxygen into the device, says study co-author Clare Grey, also of the University of Cambridge. The researchers need to find a way to keep the lithium metal from forming spindly fibers known as dendrites, which can cause batteries to short-circuit and explode, Liu says. Moreover, while this battery can tolerate water, the researchers also have to figure out how to protect it from carbon dioxide and nitrogen, which can also corrode the lithium metal, Liu adds.
The scientists detailed their findings in the Oct. 30 issue of the journal Science. The researchers have patented their work, and the intellectual property is owned by Cambridge Enterprises.
Charles Q. Choi is a science reporter who contributes regularly to IEEE Spectrum. He has written for Scientific American, The New York Times, Wired, and Science, among others.