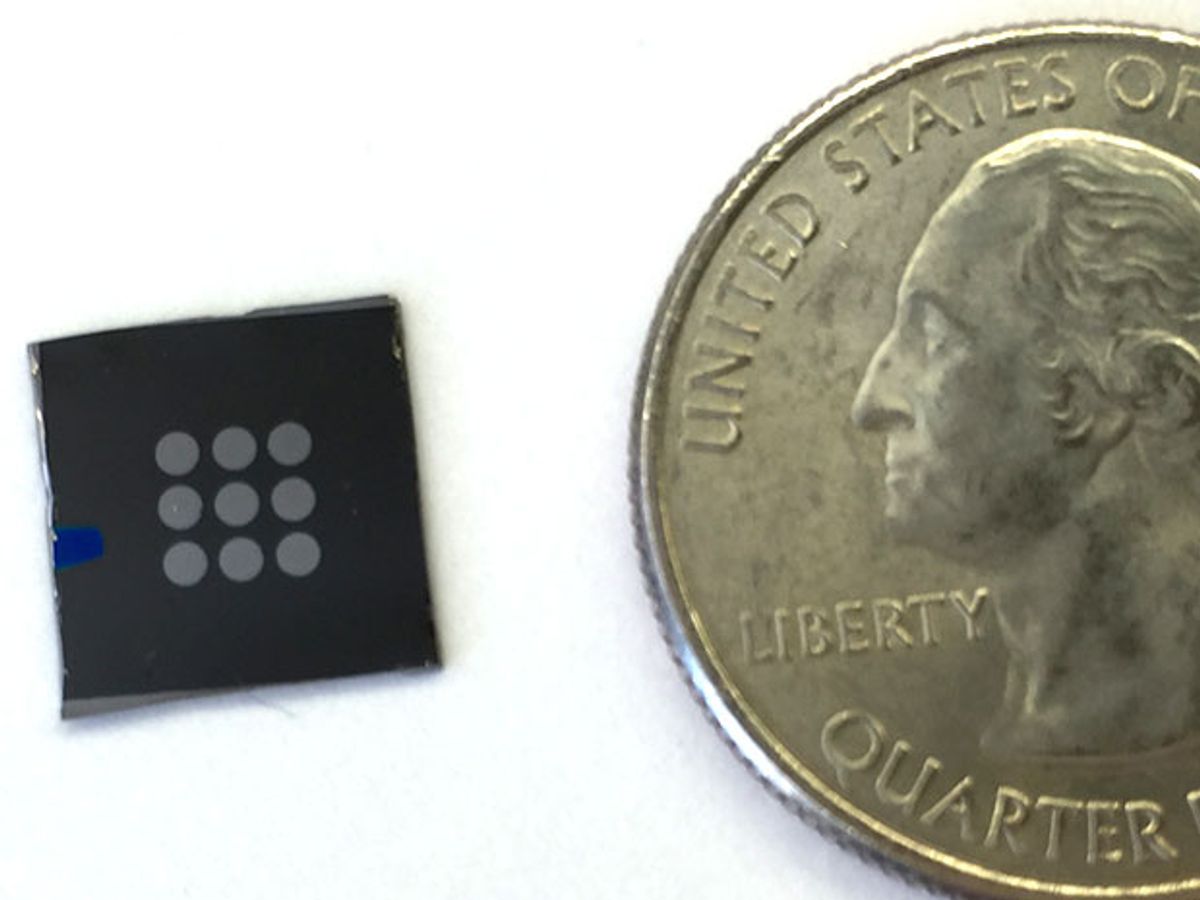
Crystals known as perovskites promise to revolutionize solar cells. Now researchers have found that they could improve fuel cells as well.
Fuel cells convert the chemical energy stored in fuels such as hydrogen into electricity. They do so by reacting the fuel with oxygen or another oxidizing agent that can strip electrons from the fuel. An electrolyte—commonly a polymer or ceramic—interposed between the fuel and oxidizer helps shuttle ions within the fuel cell.
Fuel cells are typically more efficient and environmentally friendly than heat engines, such as the internal combustion engines that usually power cars. However, fuel cells are often limited by how well their electrolytes can prevent electrons from leaking through them at the interface where the fuel and the oxidizing agent meet. Such electron conduction not only reduces fuel cell power output, but it can also lead to catastrophic fractures in the electrolyte.
Now researchers suggest that perovskites could make up a new kind of fuel cell electrolyte that can suppress electron conduction. These crystals are inexpensive and easily produced in labs, and perovskite solar cells have recently exploded in popularity in the photovoltaic world because of how quickly they have caught up to silicon-based devices in efficiency.
Fuel cell electrolytes are often chosen by how well they allow only ions to conduct through them. Instead, the researchers started with a perovskite that was very conductive to both ions and electrons.
Many perovskites are substances known as strongly correlated materials, in which each electron has a complex influence on its counterparts. By exposing a perovskite to hydrogen fuel, the scientists found that the electrons donated by the hydrogen interacted with the electrons in the perovskite. The resulting collective quantum mechanical effect suppressed electron conduction through the perovskite by nearly 100 million-fold compared to the perovskite in its pristine state.
"We have demonstrated an entirely new approach to design solid electrolytes for electrochemical energy devices," says study senior author Shriram Ramanathan, a materials scientist at Purdue University. "This opens up a new field of materials research for fuel cells and batteries."
In experiments with a perovskite samarium nickelate electrolyte, the researchers found their fuel cell reached a maximum power output of 225 milliwatts per square centimeter at 500 degrees C, which they say is comparable to the best-performing proton-conducting fuel cells in the same temperature range.
Future research will focus on developing inexpensive electrodes that can interact well with the new electrolyte, and on scaling up the system for practical power generation in applications such as vehicles and portable devices, Ramanathan says.
The scientists detailed their findings online 16 May in the journal Nature.
Charles Q. Choi is a science reporter who contributes regularly to IEEE Spectrum. He has written for Scientific American, The New York Times, Wired, and Science, among others.