To smooth out the peaks and valleys inherent in generating electric power from the sun and the wind, utility companies want massive battery farms capable of storing the surplus energy from renewable power sources for use when the sun goes down and the wind isn’t blowing. One candidate for this application is a redox flow battery that uses liquids to store and release energy.
Redox flow batteries possess a number of features that make them attractive for large-scale energy storage for power girds. For instance, their cost per kilowatt-hour is lower than the lithium-ion batteries often used in mobile devices, and their overall energy capacity can easily be expanded by adding more fluid to match a grid's growing needs.
Heretofore, they’ve been limited by low energy density—that is, energy stored per unit volume. To offer the storage needed by a local or regional power grid, they would need a lot of space, which is often limited in the cities they are intended to power. For example, the energy density of the vanadium redox-flow battery, the most developed type of redox flow battery, is roughly one-tenth that of lithium-ion batteries.
But in a paper published in the 27 November online edition of the journal Science Advances, scientists in Singapore reported that they have developed new redox flow lithium batteries whose energy densities match those of their lithium-ion counterparts. “The energy density of redox flow lithium batteries can be about eight to 10 times as high as conventional redox flow batteries,” says Qing Wang, a materials scientist at the National University of Singapore who is a member of the team that made the breakthrough.
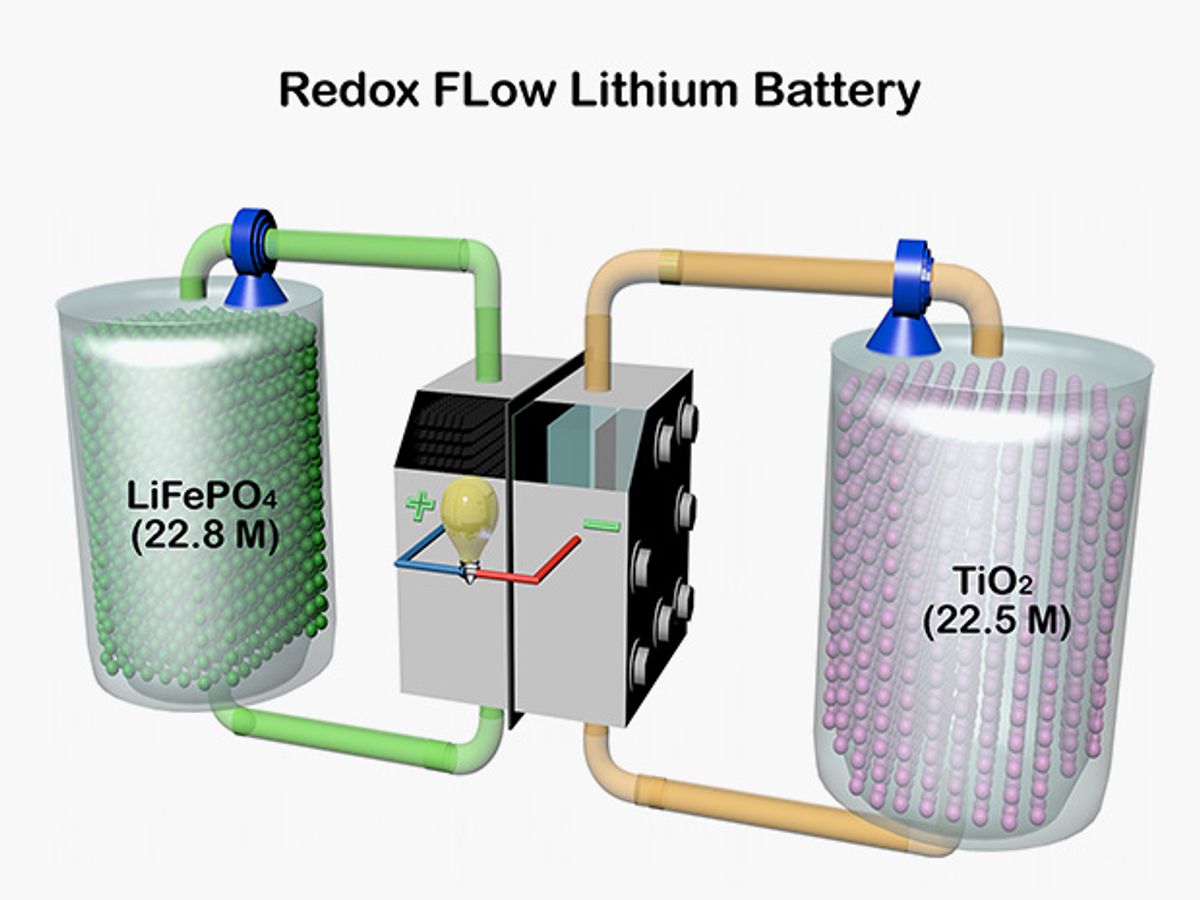
The key innovation: solid granules in the electrolyte tanks made from the same kinds of compounds that make up the anodes and cathodes in lithium-ion batteries.
Previous research had explored using solid materials in redox flow batteries. But prior approaches used viscous slurries of solid materials, which can take a lot of energy to pump through a battery’s interior. The new redox flow lithium battery keeps the solid granules stationary, and only pumps the electrolytes around.
The scientists used granules of lithium iron phosphate for the cathode material and titanium dioxide for the anode material. The granules are porous, to increase the amount of surface area available for electricity-generating chemical reactions.
One challenge the scientists faced in developing this battery was fabricating the membrane separating the electrolytes. The membrane needed to possess high permeability to lithium ions, low permeability to other chemicals, and good mechanical and chemical stability. The researchers ultimately settled on a lithium-loaded composite membrane made of the commercially available electroactive polymer Nafion, which is commonly used in fuel cells, plus polyvinylidene difluoride, a tough plastic that is resistant to flame, electricity, and attack by most chemicals.
Still, says Wang, the membrane they used is not good enough for transporting lithium ions at the scale necessary for power storage applications. Future research is needed to improve the membrane and other parts of the battery before before the improved redox flow battery can be used by utilities.
Charles Q. Choi is a science reporter who contributes regularly to IEEE Spectrum. He has written for Scientific American, The New York Times, Wired, and Science, among others.