30 September 2011—If every leaf on the planet can do it, maybe we can too. Scientists have long tried to mimic photosynthesis as a way to harness the energy in sunlight and turn it into a usable fuel, just as plants do. There have been big technical challenges for just as long, and though scientists are far from the ultimate goal, two reports published online in the journal Science yesterday describe some solutions to the obstacles.
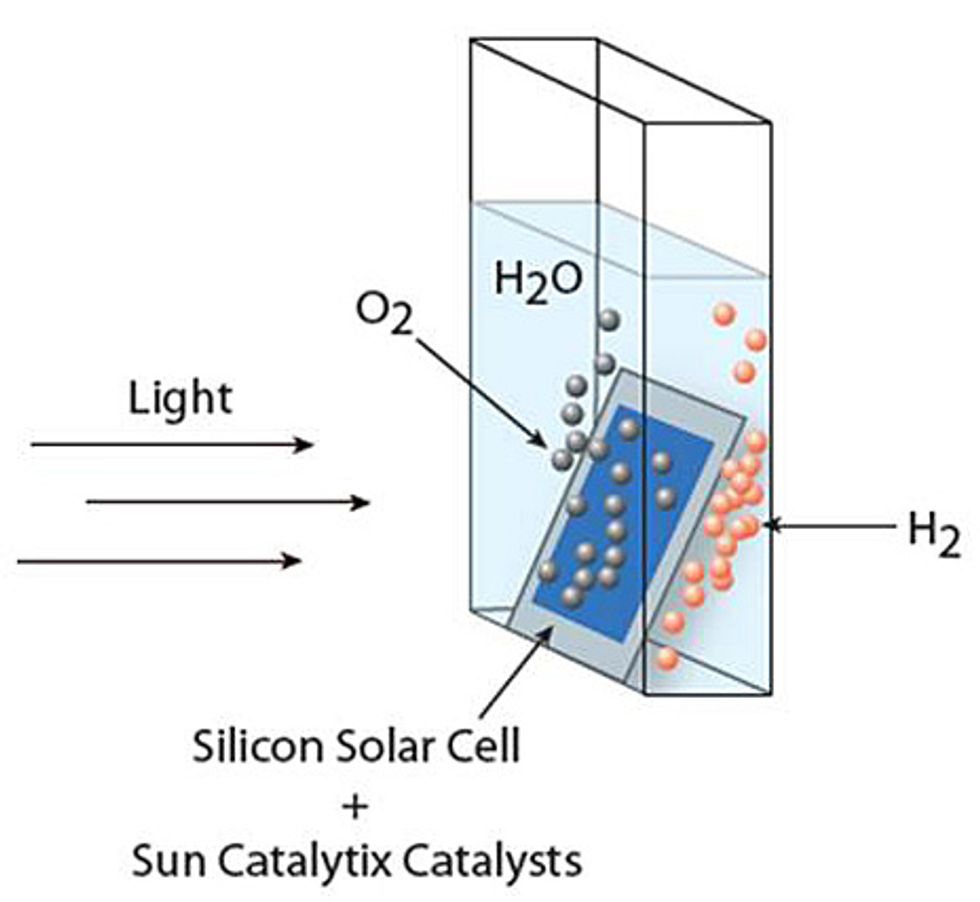
In one report, a group led by MIT chemistry professor Daniel Nocera found a new way to use light to split water molecules into oxygen and hydrogen, which could then be stored and used as a fuel. Other groups have had some success with this process before, but there were always stumbling blocks that would make it hard to scale up or commercialize, such as extremely acidic or basic conditions, expensive catalytic materials, or both. However, Nocera’s group managed to get artificial photosynthesis to work using benign conditions and cheap, abundant materials as catalysts.
Specifically, the team joined a commercially available triple-junction solar cell to two catalysts: cobalt-borate for splitting the water molecule and a nickel-molybdenum-zinc alloy to form the hydrogen gas. The water-splitting reaction achieved a sunlight-to-fuel conversion of 4.7 percent in one incarnation of the device and 2.5 percent in another. The difference between the two was that the more - efficient device housed the hydrogen-generating alloy on a mesh wired to the solar cell. The less efficient version was wireless, and the alloy was instead deposited onto the stainless-steel back of the solar cell.
It is the wireless possibility, where the entire device is self-contained, that researchers say is most exciting. "Because there are no wires, we are not limited by the size that the light-absorbing material has to be," says Steven Reece, a research scientist with Sun Catalytix (a company cofounded by Nocera) who worked on the discovery. "We can operate on the micro- or even nanoscale…so you can imagine micro- or nanoparticles, similar to the cells we’ve worked with here, dispersed in a solution." The researchers say they are still deciding what size the final product should be—anywhere from a small, leaf-sized stand-alone system that might be able to power an individual home to a much larger system that could benefit from economies of scale. Whatever size they decide on, the researchers believe such devices could help provide power in poor areas that lack consistent sources of electricity.
"As the inputs are light and water, and the output is fuel, one can certainly see the applicability of something like that to the developing world," says Thomas Jarvi, chief technology officer at Sun Catalytix.
Jarvi says the company expects to be able to bring the device to the point where a kilogram of hydrogen could be produced for about US $3. Given that a gallon of gasoline contains about the same amount of energy as 1 kg of hydrogen, as long as gas prices stay north of $3 per gallon, this would make a cost-effective fuel source.
Daniel Gamelin, a professor of chemistry at the University of Washington who works on related topics but was not involved with the new study, says the MIT and Sun Catalytix work represents an "impressive accomplishment." However, he says, it remains to be seen whether silicon is really the most desirable material to use, noting that something less susceptible to degrading by oxygen may be a better option.
"For these specific devices, there remain open questions about their long-term stability," Gamelin says. "And their efficiencies would still need to be increased substantially to be commercially viable. But there is obviously potential for improvement on both fronts. In the bigger scheme, [this research] marks important progress toward the development of truly practical solar hydrogen technologies."
The other report, published simultaneously with the hydrogen producer, demonstrated a different type of advance—a step toward using sunlight to recycle carbon dioxide. In the natural world, the sun’s energy extracts electrons from a water molecule, which then reduce CO2 into fuel (in plants, the fuel takes the form of carbohydrates). University of Illinois graduate student Brian Rosen and other scientists were able to invent a device that electroreduced CO2 to carbon monoxide at a lower voltage than previously achieved. The high voltages usually required have been a primary stumbling block in CO2 electroreduction in the past. Rosen’s group brought the voltage down by using a combination of a silver cathode and an ionic liquid electrolyte that presumably stabilized the CO2 anion. And according to Rich Masel, who led the research and is CEO of Dioxide Materials, a company working on CO2 electroreduction with the University of Illinois, this piece of the photosynthetic process could eventually lead to a way to turn captured CO2 into "syngas"—a mixture used in the petrochemical industry to make gasoline and other fuels.
The experiment "shows that one can make syngas efficiently from any source of electricity," Masel says. However, large-scale versions of the device probably won’t be demonstrated until 2018. "Presently we have demonstrated the process on the 1-centimeter-squared scale. We need to go to the million cm2 to make significant amounts of gasoline."
Work on artificial photosynthesis has ramped up considerably in recent years. In July 2010, the DOE began funding a Joint Center for Artificial Photosynthesis to the tune of $122 million over five years as part of its Energy Innovation Hubs program; it is led by Caltech professor of chemistry Nate Lewis. The center, with close to 200 members in universities and national laboratories across California, aims to build on nature’s photosynthetic design, bridging all the disciplines required, from chemical engineering to applied physics.
In an interview earlier this year, Lewis told Spectrum that progress is certainly being made, but it isn’t clear yet if the right combination of catalysts and light absorbers and everything else that goes into practical artificial photosynthetic devices has been found.
"We’re seeing light in the tunnel," he said. "We don’t know where the end of the tunnel is. It’s a curved tunnel."
This article was updated on 17 October 2011.
About the Author
Dave Levitan is a science journalist who contributes regularly to IEEE Spectrum’s Energywise blog. He recently wrote about how biology is inspiring more efficient wind power.