The U.S. Defense Advanced Research Projects Agency (Darpa) estimates that soldiers on a typical desert reconnaissance mission could cut their battery load in half by carrying portable photovoltaic cells and recharging them from the sun. Seeking solar chargers suitable for a backpack, military researchers are turning away from the inorganic semiconductors, like silicon, that rule the solar market to organic photovoltaics (PV) composed of carbon-based dyes and polymers. Organic materials could even displace the thin films expected until recently to provide PV's next generation.
Organic photovoltaics fit the bill because they weigh next to nothing, bend without breaking, and are showing rapidly improving efficiencies. Although their ability to convert photons into electricity must improve still more, the vision of solar plastics is moving rapidly toward realization, thanks to an R and D investment by the U.S. Department of Defense. "We're starting to make prototype devices to try out in the field," says Lynne Samuelson, who leads an organic PV research program at the U.S. Army's Natick Soldier Center, in Massachusetts.
Even partial success in these military programs could catapult organic PV into consumer markets for solar power, from trickle-charging coatings on portable electronics to solar shingles. As a result, venture capitalists and manufacturers are increasingly investing in organic PV. Electronics giants Siemens and STMicro-electronics have internal research efforts, while others, like Matsushita Electric Industrial, are partnering with start-ups developing organic photovoltaics.
The military's push into organic PV began four years ago at the Natick center, which is to the U.S. foot soldier what Q is to James Bond at MI6. In 2000, Natick teamed up with chemists at the University of Massachusetts Lowell, who were working on Grätzel cells, a form of organic PV named for Michael Grätzel of the Swiss Federal Institute of Technology in Lausanne. (Grätzel invented the cells 12 years ago; the team at Lowell had been assembled by the late Sukant K. Tripathy, a talented chemist who developed a process for attaching particles of titania--titanium dioxide--to plastic.) Grätzel cells mimic photosynthesis: light-sensitive organic dyes dissolved in an electrolyte absorb light and transfer energized electrons to titania nanocrystals sintered to an electrode-coated substrate.
By 1994, the best Grätzel cells generated power at 10 percent efficiency--rivaling the best commercial solar panels of the time--yet the technology languished because the liquid electrolytes were sensitive to heat and prone to leakage. For Samuelson and her colleagues at Natick, Grätzel cells had a final, fatal flaw: assembled on glass plates that could withstand the titania-sintering step at 450 �C, they were hardly appropriate for a backpack.
But over the last four years, the University of Massachusetts team, since spun off in Lowell as Konarka Technologies Inc., has addressed each flaw, and this winter it hopes to complete its first military prototypes. Konarka plans to begin selling modules by mid-2005, and with US $13.5 million in venture capital and such business partners as Eastman Chemical Co. in Kingsport, Tenn., and utility giant Electricité de France, they appear to have the resources to get there.
Konarka worked with Grätzel to develop heat-stable gel-based electrolytes, whose viscosity makes them more leakproof than liquids, and a tighter sealing material to keep the electrolyte fixed. "You can take these cells and cut them in half, and they still work," Samuelson says.
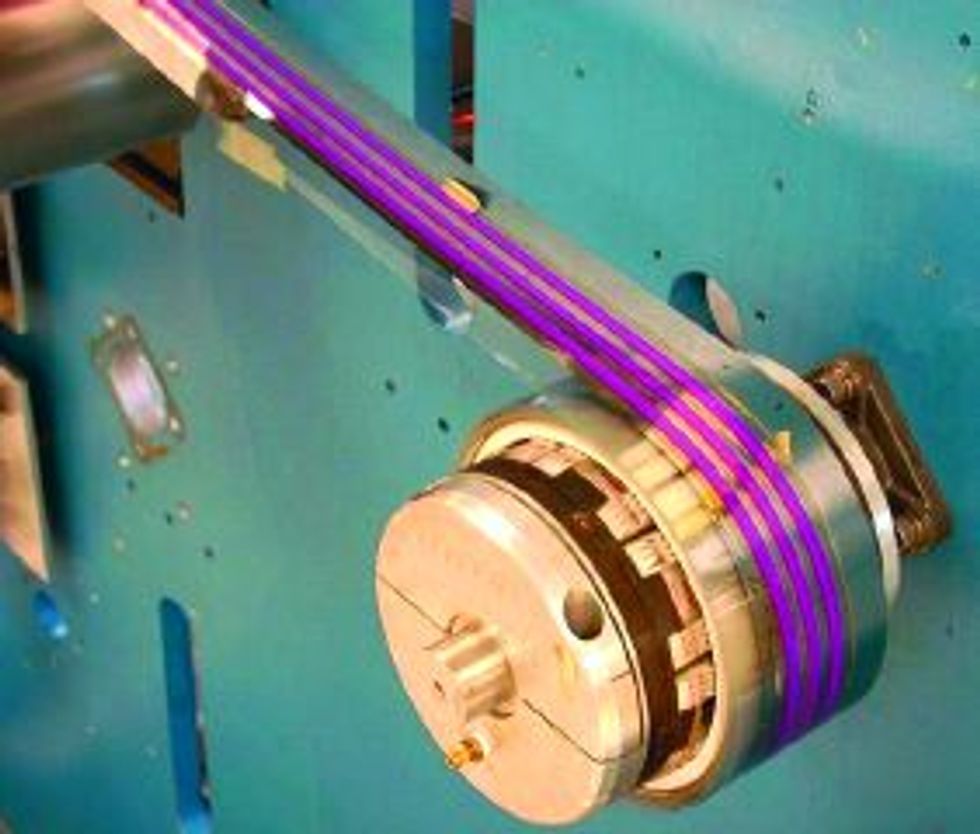
Most important, Konarka found a way to produce the cells on cheap, light, and flexible sheets of poly-ethylene terephthalate (the clear plastic of soda bottle fame) in a continuous process [see photo, "On a Roll"]. Titania particles 20-30 nm in diameter are sintered onto stainless steel or titanium foil in 1- to 2-cm-wide strips, which are then laminated onto the plastic sheet, covered with electrolyte, and capped with an electrode-coated top sheet of plastic.
According to Konarka's vice president for R and D, Russell Gaudiana, modules assembled from these cells will weigh one-third as much as the lightest flexible photovoltaics available today, which employ amorphous silicon on heat-resistant engineering polymers (those with the qualities needed to replace metals) and cost half as much to produce, at under $1 per watt. Their output could also be higher: Gaudiana says large cells rolling off its coating machines convert 6.8 percent of incident solar energy into electricity, matching the best amorphous silicon products, and could eventually achieve 16 percent. Samuelson deems Konarka's current performance "very usable" for military applications.
Of course, modules that convert more light into electricity would deliver more bang for the buck--and kilo--and that's what Darpa's program is seeking to gain from another family of organic photovoltaics: nanocomposite cells. These cells are analogous to theorganic light-emitting diodes now entering the display market [see "The Dawn of Organic Electronics," IEEE Spectrum, August 2000,pp. 29-34]; they employ mixtures of organic dyes, polymers, and nanostructures that mimic the light-absorbing pn junction of inorganic photovoltaics.
First reported by Kodak researchers in 1986, these pn mimics were stuck at a paltry 1 percent conversion efficiency until 2000. Since then, hybrids incorporating inorganic and organic nanomaterials have been found to conduct better and have achieved power conversion of better than 3.5 percent.
Like the Grätzel cells, these layered organic and nanocomposite cells are amenable to low-cost processing. Many can be assembled layer by layer with simple spray-coating techniques. Darpa thinks nanocomposite organics are poised for a step change: its goal is to push efficiency to 20 percent over five years. As many as 30 or 40 teams are rumored to be bidding for Darpa's dollars, and if the proposals pass muster, two to four of them could be off and running within months with $5 million to $10 million each--providing a major boost for the field.
Top experts and players in organic electronics are optimistic. "Today's efficiency numbers are not the end point by any stretch of the imagination," says Stephen Forrest, a physicist at Princeton University in New Jersey and a pioneer in the field. Already, "we're far beyond what's been published," says Stephen Empedocles, director of business development for the start-up Nanosys Inc., in Palo Alto, Calif.
But will low-cost organic PV ever crack the most price-sensitive market of all: rooftop panels? Today's rooftop installations are warranted to operate through 20-30 years of environmental abuse, and that's a high bar for organic electronics, which tend to be less stable than inorganic semiconductors. Several decades of warranted performance is a "tremendous requirement," says Franz Karg, global head of R and D for PV producer Shell Solar, an Amsterdam subsidiary of the Royal Dutch/Shell Group in The Hague. "Frankly, I don't expect this performance in the next 10 to 15 years from organics, if it's possible at all." Nanosys and Matsushita insist they can deliver that performance by 2007.