Sweat Sensors Will Change How Wearables Track Your Health
Your sweat may bring medical diagnostics to Fitbits and Fuelbands

Sweat, ick. It betrays our nervousness, leaves unsightly blotches on our clothes, drips down our faces, and makes us stink. Sure, it cools us when we overheat, but most of the time we think of it purely as an inconvenience.
We may soon, however, learn to like our sweat a lot more—or at least what it can reveal about our health. We’d certainly prefer giving a doctor a little sweat to being punctured for a blood test—or even providing a urine sample—as long as we didn’t have to run a mile or sit in a sauna to do it. And if sweat could provide constant updates about our bodies’ reactions to a medication, or track head trauma in athletes, we might just start to appreciate it.
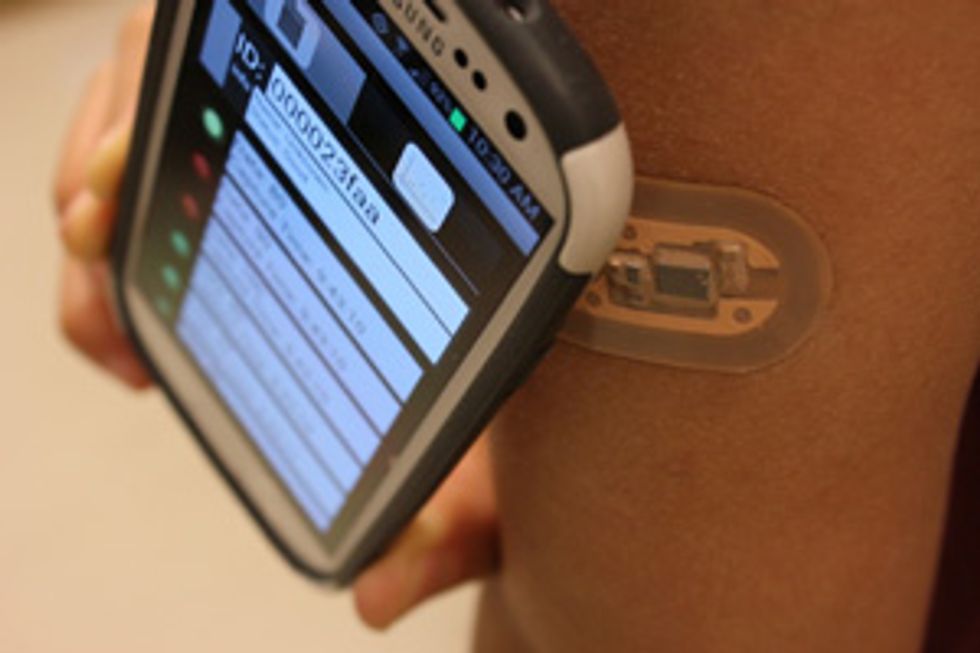
Sweat contains a trove of medical information and can provide it in almost real time. And now you can monitor your sweat with a wearable gadget that stimulates and collects it using a small patch and analyzes it using a smartphone—that is, if you visit my lab.
Using sweat to diagnose disease is not new. For decades, doctors have screened for cystic fibrosis in newborns by testing their sweat. And in the 1970s several studies tried using sweat to monitor drug levels inside the body. But in the early days of sweat diagnostics, the process of collecting it, transporting it, and measuring it was vastly more complicated than an ordinary blood test, so the technology didn’t catch on.That’s about to change. Researchers have discovered that perspiration may carry far more information and may be easier to stimulate, gather, and analyze than previously thought.
My group at the University of Cincinnati, working with Joshua Hagen and other scientists at the U.S. Air Force Research Laboratory, at Wright-Patterson Air Force Base, in Ohio, began five years ago to look for a convenient way to monitor an airman’s response to disease, medication, diet, injury, stress, and other physical changes during both training and missions. In that quest, we developed patches that stimulate and measure sweat and then wirelessly relay data derived from it to a smartphone. In 2013 the Air Force expanded on my group’s work and that of our collaborators by sponsoring the Nano-Bio-Manufacturing Consortium, in San Jose, Calif., created to accelerate the commercialization of biomonitoring devices such as sweat sensors.
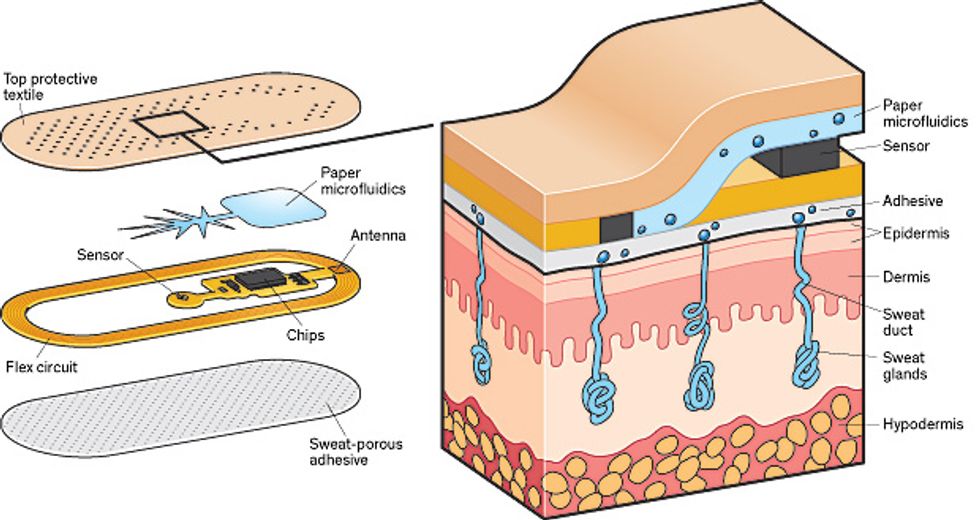
Perspiration Detective: This patch, developed at the University of Cincinnati, uses paper microfluidics to wick sweat from the skin through a membrane that selects for a specific ion, such as sodium. Onboard circuitry calculates the ion concentration and sends the data to a smartphone. The electronics within the patch are externally powered, as in an RFID chip.
My colleagues and I started by looking for something sweat could reveal that would be useful to a large number of people. We settled on monitoring physical fatigue—in particular, alerting athletes if they were about to “crash” because of overexertion or dehydration. This problem may sound mundane, but it is hard to predict. Even million-dollar athletes regularly leave competitions because of cramping, and warning of an approaching imbalance in electrolytes could prompt an athlete to take in fluids to avoid such a mishap.
With the testing of athletes in mind, we started by measuring the substances dissolved in sweat. You probably know, thanks to decades of commercials for Gatorade, that sweat is rich with electrolytes, electrically charged ions of elements like sodium, chlorine, and potassium, with concentrations from ones to tens of millimoles per liter. (In biological terms, that is actually a lot: Normally, blood has a 3.5 to 5.2 millimolar concentration of potassium. That is, it contains 3.5 to 5.2 millimoles of potassium per liter.) Ideally, we wanted to figure out the balance of electrolytes in sweat and how it correlates to the balance of electrolytes in the blood, because it is an imbalance of electrolytes in the blood that causes severe symptoms of dehydration like muscle cramping.
Measuring the saltiness of sweat doesn’t turn out to be particularly useful for monitoring athletes, because levels of sodium and chloride in sweat don’t correlate with any particular changes in blood levels. That’s because the cell membranes lining the sweat gland act as “salt pumps.” When messages from the central nervous system trigger the membranes to push negatively charged chloride ions out, they drag positively charged sodium ions with them, maintaining a neutral charge in the sweat duct. The insides of the cells become less salty than their exteriors. This imbalance draws water through the cell membranes into the sweat duct, until the sodium and chloride concentrations again match; as a result, the cells shrink until they can replenish themselves by pulling in water and salt from adjacent cells. The process repeats to create more and more sweat.
On the other hand, measuring levels of sodium and chloride in sweat is essential in diagnosing cystic fibrosis. The cells lining the upper portion of the sweat ducts normally reabsorb most of the salt that is produced by the sweat creation. (The body is smart; we need to retain those electrolytes.) But for patients with cystic fibrosis, the cells that handle that reabsorption don’t work properly, and simple benchtop equipment in a doctor’s office can detect the presence of saltier-than-normal sweat.
What’s in Sweat?
Biomarkers contained in sweat can give indications about the physical state of the body. They include electrolytes, metabolites, proteins, and amino acids. Here’s a sampling:
Electrolytes
- Sodium
- Chloride
- Potassium
- Calcium
Metabolites
- Lactate
- Creatinine
- Glucose
- Uric acid
Small Molecules
- Amino acids
- DHEA
- Cortisol
Proteins
- Interleukins
- Tumor necrosis factor
- Neuropeptides
With sodium and chloride off the table, we looked at a number of other substances in the blood whose levels increase when the body gets dehydrated and that diffuse into sweat in a more orderly way, meaning that when they appear in high concentration in sweat, they must be at a high concentration in the blood. Like sodium and chloride, these are small ionic solutes in sweat—ones that I can’t specify here, unfortunately, because of confidentiality agreements.
Although we couldn’t use them directly to gauge dehydration, we weren’t quite done with sodium and chloride. We found that the faster the sweating, the saltier the sweat (because there is less time for the body to reabsorb the sodium and chloride). Correlating the levels of electrolytes in sweat with their levels in blood isn’t exactly straightforward. That’s because their diffusion from blood into sweat is slow. So as the rate of sweating increased, the telltale substances we were tracking in the sweat became more diluted. By monitoring sodium and chloride levels, too, we could correct our sweat measurements accordingly.
Detecting sodium and chloride ions requires two things: an electrode coated with an ion-selective membrane, and a reference electrode, typically made of silver chloride. The coating for the ion-selective membrane is a standard polymer—like the plastic used to make plumbing pipes—through which ions have great difficulty penetrating, along with a special ionophore molecule that allows the passage of only one type of ion. If the ionophore is for sodium, sodium is able to easily penetrate into the polymer coating, and because sodium is a positively charged ion, a voltage of several millivolts builds up. Because the voltage of the reference electrode does not change, you can measure the total voltage of a circuit by connecting the two electrodes with a meter, calculating the voltage induced by the ion-selective membrane, and from that calculating the ion concentration. As sodium and chloride generation by sweat are interrelated, you also obtain a simple measurement of chloride.
That’s how we can find out how much salt is in sweat. Trickier is capturing the sweat quickly, getting it to the sensors, and then disposing of it, because you don’t want to hang on to old sweat and mix it with new. We decided to use paper microfluidics, the lowest-cost form of plumbing we could find that would move fluid along the patch. Pregnancy-test sticks use paper microfluidics in this way.
In our patch, the paper wicks sweat in a tree-root pattern, maximizing the collection area while minimizing the volume of paper. To keep the sweat pumping along after it passes through the sensors, these microfluidic channels direct the sweat to a superabsorbent hydrogel, such as the filler used in diapers, which pulls the sweat out of the paper and stores it. The patch can pull sweat along for several hours with the hydrogel swelling only 2 to 3 millimeters, enlarging it to hundreds of times its original volume.
We built a sodium sensor, the voltage meter, a communications antenna, the microfluidics, and a controller chip onto a patch that’s externally powered (like an RFID chip) by a smartphone. We printed it onto a flexible substrate and, with the help of researchers at the 3M Co., coated it with a sweat-porous adhesive so that it could stick to the skin. In tests, this patch performed as well as the benchtop electrolyte-sensing systems used by doctors to test for cystic fibrosis. We have had a couple of people in our research group wearing the patches for as long as a week.
Right now our industry partners are preparing to use standard flexible-electronic manufacturing processes to produce several hundred patches for more extensive human trials, which are expected to start before the end of the year. We’re also adding about a half dozen other sensors that will detect additional ions besides sodium and chloride and use them to predict things like exertion level and muscle injury or damage. The initial results look promising, and if the upcoming human trials go well, it’s not a far stretch to imagine using the patch in conjunction with the RFID-reading mats that already record marathoners’ split times to also identify runners at risk of a dangerous electrolyte imbalance.
This kind of passive patch should work great for athletes, who are usually pumping out plenty of sweat. But my colleagues and I also wanted to measure sedentary people—for example cystic fibrosis patients, who normally don’t sweat much.
The solution is to use an electrical process, called iontophoresis, which stimulates skin to produce sweat. Iontophoresis works by placing an electrically charged medication on the skin and using an electrode and a low current—less than 1 milliampere per square centimeter—to draw the medication into the skin.
Doctors have used iontophoresis for years to push anti-inflammatory drugs through the skin to reach injured tissue. And they have used it in a cystic fibrosis test for newborns to infuse pilocarpine, a medication that stimulates sweat glands, into the skin.
We’ve built the components needed to add this same capability to our patch. By carefully controlling the current that drives the iontophoresis, and therefore the absorption of pilocarpine, we can keep sweat flowing on as big or as small a spot as we want for hours, and possibly even days, at a time.
Electrolytes are by far the easiest component of sweat to measure. But metabolites—like lactate, creatinine, and glucose—shouldn’t be too much harder.
The lactate level is a great indicator of a person’s ability to cope during rigorous exercise or while on life support. Lactate, or lactic acid, is a by-product of burning glucose without oxygen. Therefore, when the body is not getting enough oxygen, it generates more lactate. Higher concentrations of creatinine and urea indicate an unhealthy kidney struggling to clear waste products from the body. In people with chronic kidney disease, so much urea is excreted with sweat that the accumulation of uric acid crystals makes the skin look frosted. And glucose monitoring, of course, is key to managing diabetes.
As yet, we have not found a way to predict exact blood levels by measuring these metabolites in sweat. So using sweat to monitor glucose levels, as desirable as that would be, is still out of reach. But being able to sense a general increase or decrease in metabolites, even without knowing their exact concentrations, can still be valuable, as Joseph Wang and his colleagues at the University of California, San Diego, recently demonstrated.
Wang’s team built a tattoolike electronic sweat sensor and had test subjects wear it during a vigorous cycling routine. Measuring a change in lactate, Wang found, might be sufficient to warn that an athlete was going to “hit the wall.” Joshua Windmiller, a former student of Wang’s, has started a company, Electrozyme, to commercialize the technology.
Metabolites like lactate in sweat are in the micromolar to millimolar range, still a relatively high biological concentration and easily measurable with a simple circuit. You again coat an electrode, but here the coating includes an enzyme specific to a particular metabolite, such as glucose oxidase or lactate oxidase. (Enzymes lower the amount of energy needed to cause a reaction.) Lactate oxidase, for example, breaks lactate into pyruvate and peroxide. The energized electrode steals two electrons from each molecule as it breaks the peroxide into oxygen and two hydrogen protons. Because lactate oxidase affects only lactate, only more lactate can generate more electrons, so any change in lactate concentration shows up as a change in current through a sensing circuit.
More work in developing this type of sensor is needed, though, because when sweat glands work really hard, they also generate their own lactate, which can skew the data. The measurement of some other metabolites with this technique, however, isn’t subject to this problem. For example, the sensors that measure current also work well for other molecules that react in the presence of an enzyme, including urea, which in addition to signaling kidney health shows a substantial increase in both blood and sweat when dehydration reaches a dangerous point.
Compared with ions and metabolites,many of the biomarkers that doctors rely on for diagnosis of stress, disease, poor nutrition, injury, and other conditions are far harder to detect because they are found in blood and sweat in mere nanomolar to picomolar concentrations (a mere billionth or trillionth by weight). But detecting their presence in sweat is nevertheless possible.
Lately, some of the hardest-to-measure biomarkers—small-protein cytokines—are generating the most excitement. Cells release cytokines under a number of circumstances, including trauma, infection, and cancer. For example, the concentration of a cytokine called interleukin 6 (IL-6) can increase up to a thousandfold during an infection.
Esther Sternberg and her colleagues at the University of Arizona recently demonstrated that several cytokines, including IL-6, have the same concentration in sweat as they do in blood. This means doctors could use sweat to diagnose a wide variety of physical and mental stresses. Right now, though, the tools needed to measure the nanomolar to picomolar concentrations of cytokines in sweat are as big as a refrigerator, or at best a suitcase. The trick here is getting the technology down to the size of a wearable gadget. My colleagues and I think that’s possible and are working toward that goal.
The basic problem is this: These biomarkers are present at such low concentrations that they can’t generate enough voltage or current themselves to overcome noise. A better strategy would be to coat an electrode with a biorecognition element—basically a biochemical puzzle piece custom designed to selectively match up to, grab, and hold the biomarker we are trying to sense. We would then apply an alternating electrical signal to the electrode. As biomarkers gather on the electrode, they should act as a barrier to electrical current, increasing the electrical impedance in a measurable way.
A more exotic and sensitive approach would be to add a molecule called a redox couple to the top of the biorecognition element. A redox couple inserted in an electrochemical process makes it easier for electrons to move from a solution to an electrode. When the biomarker binds to the biorecognition element, it changes the shape of the element, bringing the redox couple closer to the electrode, close enough to allow it to dramatically increase the flow of current. With support from the National Science Foundation and a biochemist in my lab, we recently demonstrated sensing cytokines down to a level of less than a 1-picomolar concentration using this technique.
The Air Force is interested in the possibility of measuring cytokine biomarkers to monitor extreme stresses on pilots. And it is even more interested in neuropeptide biomarkers that can give clues to the state of the brain, like one called Orexin-A, a neuropeptide biomarker that measures alertness.
In an attempt to push the limits of detection of biomarkers even further, investigators at the Air Force Research Laboratory led by Rajesh Naik are coating nanowires, nanotubes, and graphene electrodes as part of a field-effect transistor with biorecognition elements. These researchers have already built sensors capable of measuring biomarkers present at only a 1/100-picomolar concentration in simulated sweat. The issue here, for now, is that nanowires and graphene are still a bit exotic and not yet easily manufacturable.
Ultimately, sweat-sensing patches will measure multiple electrolytes, metabolites, and other biomarkers at the same time. Their designers will no doubt have to devise some clever algorithms to account for differences in the way various electrolytes, metabolites, and biomarkers migrate into sweat. But it will be worth the effort. Being able to measure multiple biomarkers might allow physicians to conduct cardiac stress tests on a treadmill without drawing blood. They could also measure the impact of drugs on the body so that dosages could be determined more precisely, as opposed to the crude estimates we use now based merely on age and body weight.
There is still work to do on the digital signal processing and algorithms needed to analyze the raw electrical measurements of biomarkers in sweat. But a physical-exertion sensor patch is a near reality, about to be tried on hundreds of people. If all goes well, we could have sweat-sensing patches—at least sensors for athletics—on the market in low volume next year. These do not have to go through a lengthy approval process with the U.S. Food and Drug Administration because they are not meant to be used for diagnosis or treatment of disease.
The second-generation patch we’re now working on in the lab is nearly complete. It includes secure Bluetooth communication, data storage, and a small microcontroller to detect higher-frequency and more complex signals from the electronic sensors on the patch. Analysis of these more sophisticated waveforms is critical for the detection of the really low-concentration biomarkers, like cytokines. Ultimately, sweat analysis will offer minute-by-minute insight into what is happening in the body, with on-demand sampling in a manner that is convenient and unobtrusive.
Researchers have understood the richness of the information carried in sweat for some 50 years, but they have been unable to take advantage of it because of the difficulty of collecting, transporting, and analyzing the samples. With the many recent advances in sensing, computing, and wearable technology providing inspiration—and with more than a little perspiration in the laboratory—we are on the verge of a true revolution in wearable diagnostics.
This article originally appeared in print as “Let Them See You Sweat.”
About the Author
Jason Heikenfeld is a professor of electrical engineering and director of the Novel Devices Laboratory at the University of Cincinnati. A founder of Gamma Dynamics, a maker of electrofluidic displays, he wrote IEEE Spectrum’s 2010 article “The Electronic Display of the Future.” An avid runner, Heikenfeld has recently focused his research on sweat—something he deals with both in and out of the lab.