Wax Fuel Gives Hybrid Rockets More Oomph
Paraffin-based fuels could allow safer, hybrid designs to rival the best liquid-fueled rockets


Since spaceflight began, there have been fewer than 5,500 launches into orbit, and only about 300 of those have carried astronauts. These endeavors have always been risky. Indeed, the failure rate for space launches over the past five decades has hovered around 8 percent.
Early aircraft were also subject to frequent accidents, but private industry invested billions in development, and these machines grew steadily safer over time. Without a mass market to drive a similar evolution, space travel has remained exceedingly dangerous. No wonder it still takes a good dollop of “the right stuff” to be an astronaut.
Soon, though, the advent of suborbital space tourism may finally do what decades of government-sponsored R&D could not. Companies such as Airbus Defence & Space, Armadillo Aerospace, Blue Origin, Virgin Galactic, and Xcor Aerospace are planning to offer suborbital flights for prices that ordinary people—or at least ordinary wealthy people—can afford.
The loss of Virgin’s Space-ShipTwo and the death of one of its pilots during a test flight on 31 October are, however, sobering reminders of the challenges that remain. If space tourism nevertheless blossoms, the technology to reach space should become increasingly safe and reliable. This would benefit a variety of space-based applications, including communications, remote sensing, and scientific research.
But that’s a big if. The well-known dangers of rocket propulsion arise simply because heaving thousands of kilograms into orbit is an extremely difficult job. Rockets must rapidly mix and react huge quantities of a fuel and an oxidizer in a combustion chamber to release enough energy each second to send prodigious amounts of hot gas flying out of the nozzle at high speed. The rocket’s various components must endure extreme low and high temperatures as well as punishing levels of vibration and stress.
In liquid-fueled rockets, a malfunction that brings fuel and oxidizer together in an uncontrolled way or causes a loss of thrust after launch can create a massive explosion, destroying the vehicle and damaging the launchpad. Orbital Sciences Corp.’s Antares launch vehicle recently experienced just such a calamity.
Solid-propellant rockets have the fuel and oxidizer already mixed and held together in a polymer binder. That reduces complexity, but it doesn’t eliminate the dangers: Cracks or imperfections in the solid fuel or its packaging can cause uncontrolled combustion and explosion, as tragically happened to the solid rocket boosters of the space shuttle Challenger in 1986 when an O-ring seal in the motor case failed.
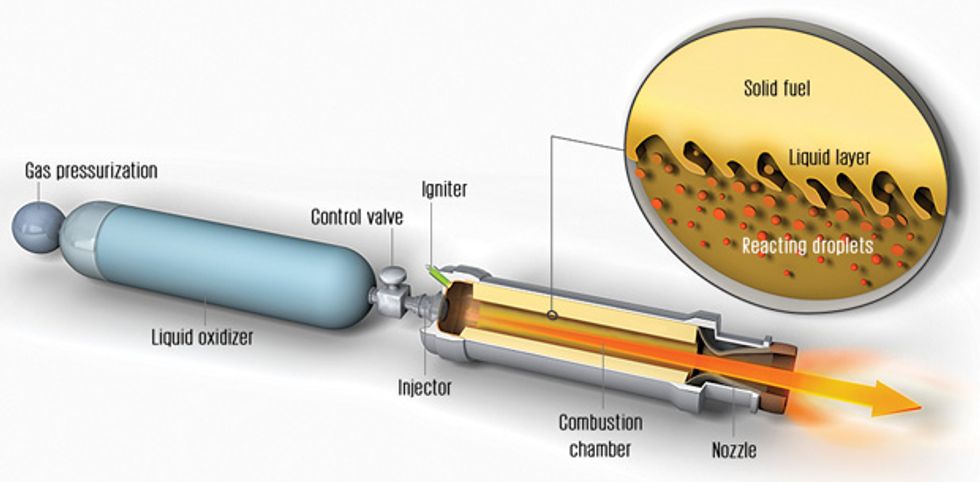
Are there no safer alternatives? One under study is the hybrid rocket. Hybrid rocket motors store the oxidizer as a liquid and the fuel as a solid, a configuration that is mechanically simple and reduces the opportunity for chemical explosion, both in flight and during ground operations. That makes hybrids safer than solid-fueled rockets. Hybrids are also more flexible because the flow of oxidizer can be controlled, meaning that the thrust can be adjusted or even shut down and restarted during flight.
These advantages prompted the designers at Scaled Composites to select a hybrid rocket motor for SpaceShipOne, the vehicle that won the Ansari XPrize in 2004 when it traveled into space twice within the span of two weeks. This was the first hybrid rocket ever used in a crewed flight. The company’s larger suborbital vehicle, SpaceShipTwo, also employs a hybrid rocket, which at the time of this writing did not appear to have caused October’s tragic accident.
Although they might seem cutting edge, hybrid rockets have in fact been around since the 1930s, when the first examples were developed in the Soviet Union. While the basic concept has always been attractive from a safety standpoint, such rockets have never really caught on in the space industry. It’s easy enough to understand why: Traditional hybrid rockets produce relatively meager thrust.
I and two colleagues, Arif Karabeyoglu and David Altman, are trying to eliminate that disadvantage through our research at Stanford University and at a small rocket-design company we founded in 1999. These efforts may one day allow hybrid rockets to become the design of choice for many different kinds of space vehicles, including those that require the best possible performance.
The fundamental innovation that could make this possible is a change in the composition of the solid fuel that’s used. Instead of the usual rubbery polymer, we use ordinary paraffin wax. Yes, wax. So when some cocky space tourist of the future tries to imitate Mercury astronaut Alan Shepard by yelling “Light this candle!” at launch, it could turn out to be a very apt description.
The overall design of a hybrid rocket is quite simple. The solid fuel is built into the combustion chamber in the form of a cylinder with one or more channels hollowed out along its axis. Once the liquid oxidizer is released from its storage tank, it becomes a gas and flows through these openings. After ignition, combustion takes place between the oxidizing gas and material evaporating from the surface of the fuel that is exposed inside the channels. The hot, high-pressure gases that result flow out of a suitably designed nozzle, generating thrust.

The problem is that fuel. The heat inside the combustion chamber causes some fuel to vaporize, at which point it readily burns with the oxidizer. But despite the intense heat in the chamber, the polymeric fuels normally used in hybrid rockets vaporize slowly, making it very difficult to produce the large amount of thrust needed for a launch into space.
To compensate, designers often try to expand the surface area of the fuel that’s exposed to combustion. That’s easy enough—just increase the number of channels. This strategy works to some extent, but if taken too far it can make the mass of fuel look like a block of Swiss cheese and may cause the fuel to fracture as it burns away.
The synthetic-rubber fuel used in the motor for SpaceShipOne required four channels for combustion to produce the needed thrust, and that caused some difficulties. As the fuel was burned, these openings became larger and larger, and the walls of solid fuel between them shrank. Toward the end of a burn, the expanding holes made the solid fuel prone to breakage. Indeed, on at least one flight of SpaceShipOne, large chunks of solid fuel went flying out of the nozzle, causing intense shaking and reducing the rocket’s performance—and making the pilot think at one point that the tail of his craft had blown off.
At Stanford, we have focused our research on ways to increase the thrust of hybrid rockets without adding these problematic extra channels. We first became interested in this challenge in the late 1990s, when the U.S. Air Force was studying some exotic cryogenic designs for hybrid rockets. One scheme would have swapped the roles of the fuel and oxidizer—its fuel was liquid hydrogen, which would flow over and burn with solid oxygen. While investigating this odd configuration, the Air Force also studied a different combination of cryogenic propellants: liquid oxygen and pentane, a hydrocarbon that’s liquid at room temperature but in this application was frozen solid, using a bath of liquid nitrogen.
The Air Force researchers found to their amazement that solid pentane burns three or four times as fast as normal fuels. These researchers graciously shared their measurements with us, and after a careful analysis, we figured out why the pentane was burning so fast. It turns out that the fuel wasn’t burning in the usual way. Rather, the heat of the combustion chamber caused a layer of liquid to form on the surface of the solid pentane. As oxidizer flowed over it, tiny droplets of liquid pentane would become entrained in the stream of gas, and the multitude of droplets offered an enormous amount of surface area for evaporation and burning.
Normal polymeric fuels also melt when they are exposed to hot combustion. But the resulting liquid is too viscous for droplets to form, so the amount of surface area over which evaporation and burning can occur is limited—hence the usual lack of oomph.
Our discovery that pentane produced tiny droplets and that they multiplied the burn rate a few times over was completely serendipitous—and very welcome. But frozen pentane itself didn’t make for a promising fuel. After all, it wouldn’t be practical to have to dip your rocket in a vat of liquid nitrogen before launch. So we started searching for something to replace frozen pentane.
We began by considering some other common hydrocarbons. Familiar examples include methane (one carbon atom per molecule), ethane (two carbons), and propane (three carbons). As the number of carbon atoms in each molecule increases, these substances become room-temperature liquids, such as pentane (five carbons), and eventually solids such as waxes and polyethylene, the material used to make milk jugs.
What we needed was something that would be solid at room temperature and produce a low-viscosity liquid when it melted. It would also have to be strong enough to withstand the high-temperature, high-pressure, high-vibration environment of a rocket motor’s combustion chamber.
The trick was to pick a hydrocarbon molecule with the right molecular weight. At high molecular weights, the liquid form of the hydrocarbon would be too viscous for droplets to form readily. At low molecular weights, these hydrocarbons are either gases, liquids, or soft solids, much too weak to withstand the rigors of the rocket’s combustion chamber. In between, though, are some Goldilocks substances with anywhere from 25 to 50 carbon atoms per molecule, which are robust enough structurally and, as anyone who has ever watched the top of a candle knows, produce low-viscosity liquids when they melt: paraffin waxes.
The kind of paraffin wax we use is sometimes called sculptor’s wax or hurricane wax. It’s surprisingly strong—something I can attest to firsthand, having had to use a sledgehammer on a few occasions to break it up and extract it from spent fuel cartridges.
Fabricating, handling, and transporting traditional solid-rocket propellants is usually very costly, but a paraffin-based fuel would be easy to deal with in all those regards. It would be nontoxic, and indeed, it wouldn’t be hazardous at all. What’s more, the complete combustion of this fuel with oxygen produces no pollutants. The by-products are simply carbon dioxide and water. A more benign, easier-to-use rocket fuel could hardly be imagined.
With mounting enthusiasm for this novel fuel, we built a laboratory-scale hybrid rocket motor that could run off gaseous oxygen and several fuels, including paraffin wax. To our delight, we found that paraffin’s operational characteristics matched our theoretical predictions: It burned significantly faster than conventional fuels (measured by how fast the surface of the fuel burns away), giving greater thrust.

How well did it do? To appreciate the answer, you need to understand how rocket engineers measure performance. You can’t use just thrust as your metric: A big rocket with poor performance could produce more thrust than a small one that performs very well. Instead, you need to evaluate thrust in a way that takes into account the amount of propellant used each second. Rocket designers do that with a quantity called specific impulse, which is directly proportional to the speed of the gas exiting the rocket nozzle. (The higher the speed, the better.)
An equally important parameter is the sheer mass of fuel that can be stored on board a motor of a given size. A high-density fuel helps, but that’s not the only consideration. In particular, the extra combustion channels required to generate adequate thrust in traditional hybrid motors reduce the volume of fuel that can be stored. And not all that fuel can be burned anyway, because that would cause the walls of fuel separating the combustion channels to break up. Together, these constraints lead to a substantial loss of performance in traditional hybrids.
Our very first laboratory prototype, a tiny motor just 5 centimeters in diameter, burned fuel three to four times as fast as typical hybrid rocket motors. Having demonstrated that, we were convinced that a wax-based hybrid rocket requiring just a single channel for combustion could compete favorably with conventional solid or liquid systems.
To further test the feasibility of a wax rocket, we and our colleagues at NASA’s Ames Research Center, in nearby Mountain View, Calif., soon built a motor five times as large, which operated with combustion-chamber pressures and fuel-consumption rates that were similar to those of rockets in commercial use. It, too, burned its fuel three to four times as fast as conventional hybrids.
To develop this technology for commercial use, we then formed a company called Space Propulsion Group, based in Palo Alto, Calif. Over the past eight years, our company has successfully conducted more than 40 test firings of a liquid-oxygen, paraffin-fueled hybrid motor that is 28 centimeters in diameter and produces 25,000 newtons of thrust. More recently, Space Propulsion Group has been testing a motor that is 56 cm in diameter, one capable of 100,000 newtons of thrust—enough to lift more than 10 metric tons. The result of all our trials—which at one point included a motor exploding—is that our designs are now very reliable and produce very high specific impulse.
Over the same period, we have been working with Greg Zilliac at NASA Ames to develop a large, wax-based hybrid that uses nitrous oxide in place of liquid oxygen, an effort known as the Peregrine project. It has taken several years and a major motor failure to finally come up with a design that performs well.
These tests aren’t just meant to show how big a motor we can make. We need them to achieve certain design goals whose success is hard to predict from theory. You see, hybrid rocket motors, while simple in principle, have some subtle complications in practice. In particular, they are subject to certain low-frequency instabilities, along with high-frequency instabilities common to all kinds of rockets.
Thanks to years of fine-tuning, the latest motor tests of Space Propulsion Group and NASA Ames have shown excellent stability. Despite this progress, the detailed physics of what goes on inside a hybrid rocket—combustion that is just as intense as that of any other kind of rocket—remain something of a mystery, which is why we continue to perform lots of experiments.
How good could such hybrid rockets get? Russia’s kerosene-fueled RD-180, which powers the Atlas V launch vehicle, is the gold standard for heavy lifting today. With enough investment, hybrid motors could be developed that would approach that engine’s impressive specific impulse at a fraction of the cost.
The hybrid-rocket concept is now more than 80 years old. For most of that time the idea has languished, but in the last decade there have been important technical advances and growing interest around the world. Research into hybrid rockets is now going on in Brazil, France, Germany, Israel, Italy, Japan, Norway, and South Korea. In the United States, such work is taking place at the Aerospace Corp., in El Segundo, Calif., as well as at NASA, Penn State, Purdue, and of course, at Space Propulsion Group and Stanford, where my colleagues and I have been working.
So there’s every reason to expect that hybrid-rocket technology will continue to improve in coming years. Perhaps paraffin-based fuels and other refinements to this old concept will eventually bring the long-sought improvements in safety, cost, and performance needed to support routine access to space.
This article was modified on 24 November 2014.
About the Author
Brian J. Cantwell is a professor in Stanford University's department of aeronautics and astronautics, where he and colleagues have been studying paraffin-fueled hybrid rockets. As a kid, he had attempted to build his own rockets, but his parents forbade such activities—for good reason. “What I was building would have blown up right away,” he admits. His rockets today, despite being vastly bigger, are considerably safer.